This study aimed to identify a new echocardiographic index to detect coarctation of the aorta in neonates and infants. The researchers measured dimensions of the aortic arch in 63 patients with coarctation and 23 controls. They found that while ascending and descending aorta diameters were similar, the transverse arch was smaller in patients. Distances between great vessel origins were longer in patients than controls. The ratio of the subclavian artery diameter to the distance between the carotid and subclavian arteries, termed the carotid-subclavian artery index, was significantly smaller in patients. Using a cutoff of 1.5, the index showed high sensitivity and specificity for detecting coarctation in neonates and infants.
![Carotid-Subclavian Artery Index: New
Echocardiographic Index to Detect Coarctation in
Neonates and Infants
Ali Dodge-Khatami, MD, PhD, Stephanie Ott, MD, Stefano Di Bernardo, MD, and
Felix Berger, MD
Division of Cardiovascular Surgery and Congenital Cardiology, University Children’s Hospital, Zürich, Switzerland, and Clinic for
Congenital Heart Diseases, Deutsches Herzzentrum, Berlin, Germany
Background. In neonates and young infants (less than 3
months), coarctation may be missed or underestimated
by echocardiography, especially with a patent ductus
arteriosus or severe concurrent illness. A reliable nonin-
vasive screening tool for coarctation would be useful for
these patients.
Methods. From 1997 to 2003, echocardiographic evalu-
ation was performed in 63 consecutive patients with
coarctation (47 neonates and 16 infants) as well as in 23
controls (16 neonates and 7 infants). End-systolic mea-
surements were obtained from 12 different sites of the
aortic arch.
Results. In patients, the diameters of the ascending and
descending aorta were comparable to controls, but the
dimensions of the transverse arch were significantly
smaller. The distances between the origins of the great
vessels were longer in patients with coarctation than in
controls. The ratio of the aortic arch diameter at the left
subclavian artery, to the distance between the left carotid
artery and the left subclavian artery, which we propose as
the carotid-subclavian artery index, was significantly
smaller in patients with coarctation. A cut-off point at 1.5
showed a sensitivity of 97.7% and 94.7%, and a specificity
of 92.3% and 100%, for neonates and young infants,
respectively. The positive predictive value to have coarc-
tation was 97.7% and 100%, for neonates and infants,
respectively.
Conclusions. The carotid-subclavian artery index is a
simply obtainable noninvasive screening parameter,
showing high sensitivity and specificity for coarctation,
and may be useful in unstable patients or in those with a
patent ductus arteriosus in which coarctation may be
overlooked.
(Ann Thorac Surg 2005;80:1652–8)
© 2005 by The Society of Thoracic Surgeons
Coarctation of the aorta is a very common congenital
heart malformation that occurs in approximately
5% of all congenital heart diseases [1]. It is frequently
associated with other abnormalities such as tubular hy-
poplasia of the aortic arch (63%), left ventricular outflow
obstruction (40%), bicuspid aortic valve (40%), ventricu-
lar septal defect (28%), and atrial septal defect (12%). It is
defined as a narrowing of the aorta immediately distal to
the origin of the subclavian artery. In most cases, a ridge
protrudes into the lumen of the vessel from the posterior
and lateral walls. In older children, clinical manifesta-
tions range from mild clinical symptoms such as hyper-
tension in the upper extremity, a systolic murmur, or
diminished femoral pulses, and echocardiographic diag-
nosis is straightforward [2]. In newborns or young in-
fants, the presentation is often more severe, in the form
of shock or severe congestive heart failure. A concomi-
tant large patent ductus arteriosus (PDA) may render the
diagnosis difficult, thus delaying surgical intervention
until after the ductus closes [3]. In these situations, an
easily measurable, yet sensitive and specific parameter
would be useful, to reliably screen for and diagnose
coarctation in all neonates and young infants. Impor-
tantly, the timing of diagnosis should be established before
closure of a patent ductus arteriosus to avoid deterioration
of cardiac function and global systemic perfusion.
This study aims at finding a noninvasive echocardiog-
raphy parameter to predict coarctation, independent of
clinical status or other confounding factors relating to the
patient.
Material and Methods
Approval for this study was given by our Institutional
Review Board, and informed parent consent was obtained
systematically. Between January 1997 and February 2003,
preoperative echocardiographic studies and demographics
of 63 consecutive neonates and young infants with coarcta-
tion who underwent corrective cardiac surgery at our hos-
pital were recorded. Young infants were included until an
age of 3 months. Echocardiographic investigations were
performed by two cardiologists (S.D.B. and F.B.) with the
Accepted for publication April 25, 2005.
Address correspondence to Dr Dodge-Khatami, Division of Congenital
Cardiovascular Surgery, Children’s University Hospital Zürich, Stein-
wiesstrasse 75, 8032 Zürich, Switzerland; e-mail: ali.dodge-
khatami@kispi.unizh.ch.
© 2005 by The Society of Thoracic Surgeons 0003-4975/05/$30.00
Published by Elsevier Inc doi:10.1016/j.athoracsur.2005.04.041
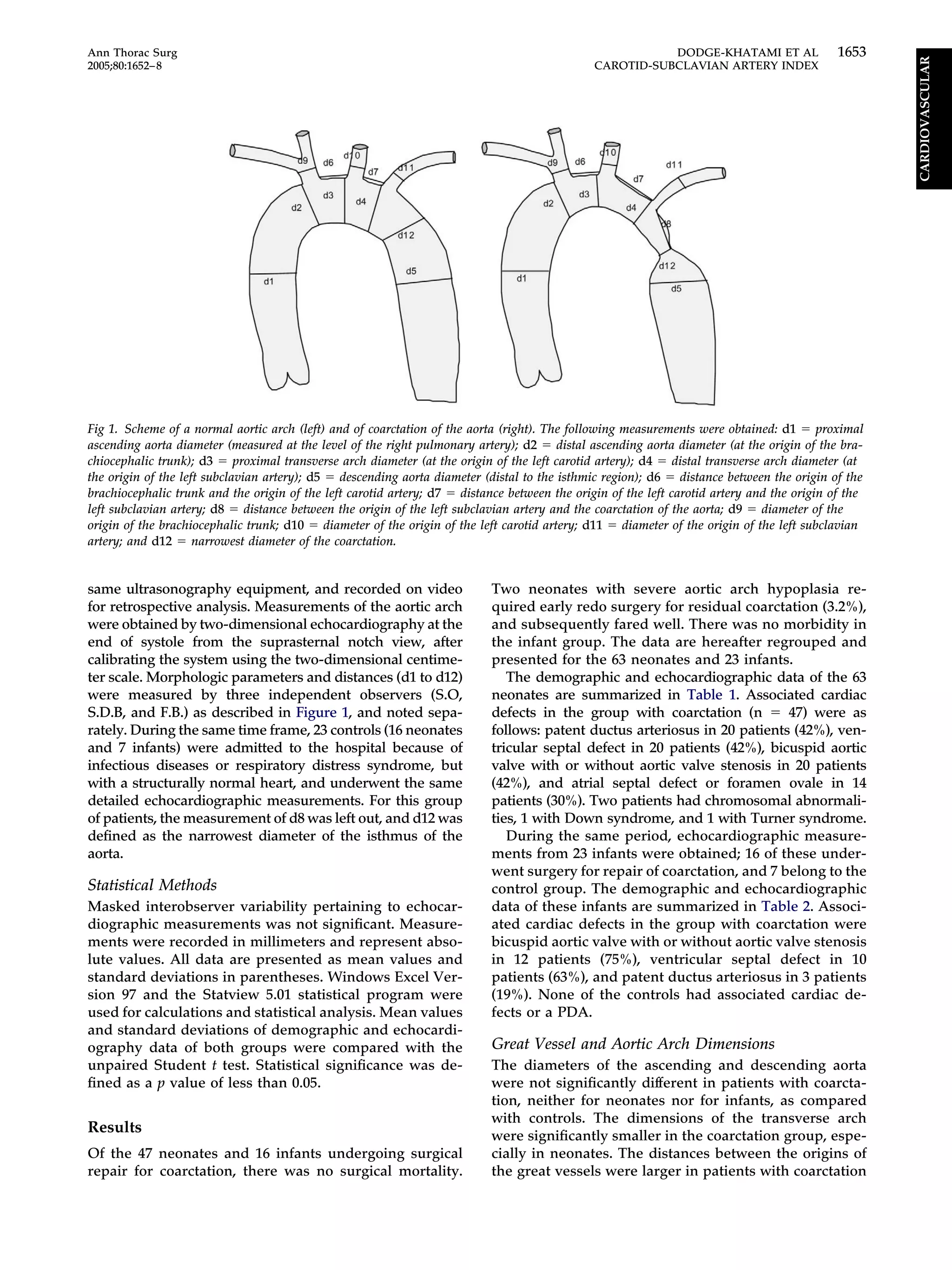
CARDIOVASCULAR](https://image.slidesharecdn.com/a1371900-ea85-4211-a52f-11ae4a80a842-161219234510/75/CSI-1-2048.jpg)
![than in controls, both in neonates and infants: the mean
distance from the brachiocephalic trunk to the carotid
artery (d6) in neonate patients with coarctation was 2.8
mm, compared with 1.5 mm in controls (p ϭ 0.0013). In
infants, the distance in patients with coarctation was 3.9
mm, compared with 2 mm in controls. The mean distance
from the left carotid artery (LCA) to the left subclavian
artery (LSA [d7]) in the neonate group with coarctation
was 7.32 mm, compared with 2.37 mm in neonate controls
(p Ͻ 0.0001). In infants, the mean distance from the LCA
to the LSA (d7) was 7.27 mm in those with coarctation,
compared with 2.67 mm in controls (p Ͻ 0.0001) (Fig 2).
The diameters of the great vessels were larger in the
coarctation group for neonates and infants; however,
significant increases were found in d10 only. Upon sub-
group analysis of patients with associated intracardiac
shunts or a PDA, there was no significant difference in
great vessel or arch dimensions, as compared with pa-
tients without associated defects.
To have a comparative parameter, we calculated the
ratios d1 to d7, d3 to d7, and d4 to d7. These indices were
proportionally significantly smaller in coarctation pa-
tients, when compared with either control neonates or
control infants (Table 3).
We used these ratios, d1/d7, d3/d7, and d4/d7, to find
predictive accuracy of two-dimensional echocardiogra-
phy in the diagnosis of coarctation for neonates, as well
Table 2. Demographic Data and Variables in Infants:
Coarctation and Controls
Infants
Coarctation
Patients
n ϭ 16
Controls
n ϭ 7 p Value
Demographic data
Age (days) 75 (34) 55 (12) 0.1318
Weight (kg) 4.43 (1.38) 4.45 (0.64) 0.9642
Length (cm) 56 (6) 55 (3) 0.6168
Body surface (m2
) 0.23 (0.07) 0.24 (0.02) 0.6967
Further measurements
Shortening fraction
of LV (%)
34 (6) 38 (5)
Gradient maximum
at COA (mm Hg)
48 (26)
Flow velocity
maximum at COA
(cm/s)
319 (116) 121 (12)
Aortic dimension
d1 (mm) 7.8 (1.1) 8.2 (2.2) 0.5576
d2 (mm) 6.8 (1.1) 7.4 (1.8) 0.2868
d3 (mm) 5.5 (1.4) 6.6 (0.8) 0.0639
d4 (mm) 4.5 (0.9) 6.3 (0.9) 0.0003
d5 (mm) 7.3 (1.9) 6.5 (0.8) 0.3054
d6 (mm) 3.9 (1.8) 2.0 (0.6) 0.0133
d7 (mm) 7.3 (2.4) 2.7 (0.8) Ͻ 0.0001
d8 (mm) 5.6 (2.5)
d9 (mm) 4.7 (1.2) 4.4 (0.5) 0.4945
d10 (mm) 3.3 (0.8) 2.4 (0.2) 0.0090
d11 (mm) 2.5 (0.5) 2.4 (0.2) 0.6439
d12 (mm) 2.3 (0.8) 5.6 (0.9) Ͻ 0.0001
Mean values are given, followed by standard deviation in parentheses.
COA ϭ coarctation; LV ϭ left ventricle.
Table 3. Ratios of Aortic Arch Dimensions to Great Vessel
Distances: Coarctation and Controls
Coarctation
Patients
n ϭ 63
Controls
n ϭ 23 p Value
Neonates 47 16
Index d1/d7 1.13 (0.83) 3.56 (1.55) Ͻ 0.0001
Index d3/d7 0.98 (0.87) 3.38 (1.43) Ͻ 0.0001
Index d4/d7 0.76 (0.86) 2.95 (1.24) Ͻ 0.0001
Infants 16 7
Index d1/d7 1.17 (0.43) 3.17 (0.83) Ͻ 0.0001
Index d3/d7 1.04 (0.43) 2.94 (0.88) Ͻ 0.0001
Index d4/d7 0.81 (0.29) 2.66 (0.78) Ͻ 0.0001
Mean values are given, followed by standard deviation in parentheses.
Table 1. Demographic Data and Variables in Neonates:
Coarctation and Controls
Neonates
Coarctation
Patients
n ϭ 47
Controls
n ϭ 16 p Value
Demographic data
Age (days) 12 (10) 16 (12) 0.15
Weight (kg) 3.0 (0.6) 3.2 (0.9) 0.37
Length (cm) 50 (7) 50 (4) 0.90
Body surface (m2
) 0.20 (0.02) 0.20 (0.04) 0.52
Further measurements
Shortening fraction
of LV (%)
34 (9) 36 (7)
Gradient maximum
at COA (mm Hg)
31 (18)
Flow velocity
maximum at COA
(cm/s)
267 (80) 130 (28)
Aortic dimension
d1 (mm) 6.8 (1.5) 7.5 (1.3) 0.0965
d2 (mm) 5.6 (1.1) 7.1 (1.2) Ͻ 0.0001
d3 (mm) 4.3 (1.0) 6.2 (1.3) Ͻ 0.0001
d4 (mm) 3.4 (0.8) 5.9 (1.4) Ͻ 0.0001
d5 (mm) 6.2 (1.4) 5.9 (1.1) 0.3227
d6 (mm) 2.8 (1.5) 1.5 (0.4) 0.0013
d7 (mm) 7.3 (3.0) 2.4 (0.8) Ͻ 0.0001
d8 (mm) 3.6 (1.6)
d9 (mm) 4.1 (0.9) 3.8 (1.1) 0.2494
d10 (mm) 2.8 (0.6) 2.4 (0.5) 0.0174
d11 (mm) 2.2 (1.2) 2.2 (0.4) 0.9052
d12 (mm) 2.1 (0.9) 5.0 (1.1) Ͻ 0.0001
Mean values are given, followed by standard deviation in parentheses.
COA ϭ coarctation; LV ϭ left ventricle.
1654 DODGE-KHATAMI ET AL Ann Thorac Surg
CAROTID-SUBCLAVIAN ARTERY INDEX 2005;80:1652–8
CARDIOVASCULAR](https://image.slidesharecdn.com/a1371900-ea85-4211-a52f-11ae4a80a842-161219234510/75/CSI-3-2048.jpg)
![as for infants. To facilitate the recognition of coarctation,
we defined the index d4/d7 as the carotid-subclavian
artery index. This ratio was significantly smaller in the
coarctation group in neonates and infants, compared
with their respective controls. If the cut-off point for the
carotid-subclavian artery index is fixed at 1.5, there is a
sensitivity of 97.7% and a specificity of 92.3% for a
neonate to have coarctation, with a positive predictive
value of 97.7%, and a negative predictive value of 92.3%.
With a similar cut-off for the carotid-subclavian artery
index in infants, our data show a sensitivity of 94.7% and
a specificity of 100%. The positive predictive value is
100%, and the negative predictive value 90.9% (Table 4).
Regarding neonates only, an index d4/d7 below 2 gives a
very specific and sensitive result, but when infants are
included, a d4/d7 index below 1.5 gives the most accurate
results taking both age groups into consideration.
Comment
Since the early 1980s, the method of diagnosis for coarc-
tation has changed from using clinical data, with or
without preoperative catheter confirmation, to relying
almost exclusively on echocardiography [4]. Echocardi-
ography can allow noninvasive assessment of the aortic
arch, identification of the narrowing at the aortic isthmus,
flow measurement, and determination of the instant
gradient over the coarctation [5–7]. However, a signifi-
cant number of patients with coarctation are not properly
diagnosed during the neonatal period [5, 6]. That may be
due to patent ductus arteriosus without flow acceleration
at the isthmus of the aorta, to poor image quality, or to a
location further downstream in the descending aorta.
Furthermore, clinical judgment may be impaired in situ-
ations with diminished contractility of the left ventricle
and poor cardiac output, or other reasons such as infec-
tion or breathing artifacts [8]. Another potential problem
is, that even with the use of Doppler flow assessment in
the descending aorta, the anatomic severity of coarcta-
tion cannot always be assessed [2, 9–11]. Other authors
have tried to find a reliable echocardiographic parameter
to predict aortic coarctation in the newborn using mor-
phologic measurements, including aortic arch diameters
at different sites, calculations and comparison of diame-
ter ratios, or measurements of distances between the
great vessels of the aortic arch [12, 13]. That has to date
not given satisfying results to clearly identify a coarcta-
tion in difficult situations, and too many diagnoses have
gone unrecognized.
The study by Morrow and coworkers [12] enforces our
results, reporting significant alterations in the dimen-
sions of arch diameters, although by invasive angiogra-
Table 4. Sensitivity, Specificity, Positive and Negative Predictive Values According to Cut-Off
Sensitivity % Specificity % Positive Predictive Value % Negative Predictive Value %
Neonates
Index d1/d7 Ͻ 1.0 59.09 100.0 100.0 41.93
Index d1/d7 Ͻ 1.5 88.63 100.0 100.0 72.22
Index d1/d7 Ͻ 2.0 97.72 92.30 97.72 92.30
Index d1/d7 Ͻ 2.5 100.0 69.23 91.66 100.0
Index d3/d7 Ͻ 1.0 84.09 100.0 100.0 65.00
Index d3/d7 Ͻ 1.5 50.00 92.30 95.65 35.29
Index d3/d7 Ͻ 2.0 97.72 84.61 95.55 91.66
Index d3/d7 Ͻ 2.5 97.72 61.53 89.58 88.88
Index d4/d7 Ͻ 1.0 97.72 100.0 100.0 92.85
Index d4/d7 Ͻ 1.5 97.72 92.30 97.72 92.30
Index d4/d7 Ͻ 2.0 97.72 97.72 97.72 90.00
Index d4/d7 Ͻ 2.5 100.0 53.84 88.00 100.0
Infants
Index d1/d7 Ͻ 1.0 52.63 100.0 100.0 52.63
Index d1/d7 Ͻ 1.5 89.47 100.0 100.0 83.33
Index d1/d7 Ͻ 2.0 84.21 90.00 94.11 75.00
Index d1/d7 Ͻ 2.5 94.73 90.00 94.73 90.00
Index d3/d7 Ͻ 1.0 63.15 100.0 100.0 58.82
Index d3/d7 Ͻ 1.5 84.21 100.0 100.0 76.92
Index d3/d7 Ͻ 2.0 89.47 90.00 94.44 81.81
Index d3/d7 Ͻ 2.5 100.0 80.00 90.47 100.0
Index d4/d7 Ͻ 1.0 89.47 100.0 100.0 83.33
Index d4/d7 Ͻ 1.5 94.72 100.0 100.0 90.90
Index d4/d7 Ͻ 2.0 100.0 80.00 90.47 100.0
Index d4/d7 Ͻ 2.5 100.0 50.00 79.16 100.0
1655Ann Thorac Surg DODGE-KHATAMI ET AL
2005;80:1652–8 CAROTID-SUBCLAVIAN ARTERY INDEX
CARDIOVASCULAR](https://image.slidesharecdn.com/a1371900-ea85-4211-a52f-11ae4a80a842-161219234510/75/CSI-4-2048.jpg)
![phy. They found no differences between patients and
controls concerning the descending aorta and left sub-
clavian artery diameters, but demonstrated that the
length of the transverse arch between the LCA and LSA
was significantly increased in patients with coarctation
[12]. Our results support his findings and add a useful
and reproducible index, with the use of a noninvasive
diagnostic tool. Nihoyannopoulos and associates [14]
assessed the predictive accuracy of two-dimensional
echocardiography in defining aortic arch obstruction.
Using viewing of the aortic arch only, the overall sensi-
tivity of the method was only 88%. They found two-
dimensional echocardiography to be more specific than
sensitive for the prediction of aortic arch obstruction,
noting that with a low origin of the LSA, particular
attention should be paid to the visualization of the
isthmus [14].
Contrary to our findings, Aluquin and coworkers [13]
found the distal ascending root diameter and descending
aorta to be significantly larger in patients with coarcta-
tion. Our data show that the proximal and distal diame-
ters of the ascending aorta are smaller in patients with
coarctation, and that the diameter of the descending
aorta is larger in coarctation patients, either due to
increased resistance before the stenosis or to post-
stenotic dilatation from turbulent flow. Nevertheless, our
data concur with theirs regarding the transverse arch,
which was notably longer in the coarctation group, as
compared with controls.
Excluding older invasive angiographic studies, newer
noninvasive modalities to accurately assess and diagnose
coarctation in the younger population exist, and are both
reliable and reproducible [2, 15]. These include axial,
multiplanar computed tomography scan and magnetic
resonance imaging, which are more expensive, cumber-
some, and could require anesthesia and intubation in the
newborn and infant population.
Because of the significant decrease in diameter of the
distal transverse aortic arch just before the LSA (d4) in
patients with coarctation, and the significant prolonga-
tion of the distance from the origin of the LCA to the
origin of the LSA (d7), we found it useful to use these two
variables as part of the carotid-subclavian artery index.
Therefore, we propose the carotid-subclavian artery in-
dex, where the diameter of the transverse arch at the
origin of the LSA (d4), is put in ratio to the distance from
the origin of the LCA to the origin of the LSA (d7), as a
screening tool for coarctation. In neonates and young
infants with coarctation, the carotid-subclavian artery
index yields a sensitivity of 97.7% for neonates and 94.7%
for infants, using a cut-off point below 1.5. The longer the
distance (d7) and the smaller the diameter of the aortic
arch at the origin of the LSA (d4), the smaller the
carotid-subclavian artery index, and the higher the pre-
dictability of coarctation. These findings remain valid
regardless of the presence or absence of an associated
intracardiac shunt or PDA.
Study Limitations
The results of our study are to be taken into the perspec-
tive of a retrospective design and its limitations. To
achieve validity, the carotid-subclavian artery index
should be prospectively assessed in patients with only
mild hypoplasia of the aortic arch, with or without
coarctation. Also, the numbers are relatively small, re-
ducing the power of the finding. To establish the useful-
ness of the carotid-subclavian artery index as a screening
tool for coarctation, a prospective study with a greater
population of newborns and infants is needed, both with
and without coarctation.
In conclusion, the carotid-subclavian artery index is
a simple screening parameter, readily obtained, and
standardized from two-dimensional echocardiography
visualization of the aortic arch. It shows high sensitiv-
ity and specificity for coarctation in our population of
newborns and infants with a cut-off point below 1.5,
independently of concomitant intracardiac or extracar-
diac shunts. In difficult subsets of patients with a large
PDA and severe concurrent illness with hemodynamic
instability, measuring the carotid-subclavian artery
Fig 2. Echocardographic images of two different aortic arches with a large distance between the left carotid artery and the left subclavian ar-
tery and significant narrowing of the transverse arch. Calculation of the carotid-subclavian index is highly specific for the presence of coarcta-
tion. (AAO ϭ ascending aorta; LCA ϭ left carotid artery; LSA ϭ left subclavian artery; TAA ϭ transverse aortic arch; Tr. brach. ϭ bra-
chiocephalic trunk.)
1656 DODGE-KHATAMI ET AL Ann Thorac Surg
CAROTID-SUBCLAVIAN ARTERY INDEX 2005;80:1652–8
CARDIOVASCULAR](https://image.slidesharecdn.com/a1371900-ea85-4211-a52f-11ae4a80a842-161219234510/75/CSI-5-2048.jpg)
![index may lead to earlier diagnosis and subsequent
surgical correction, before ductal closure and dimin-
ished cardiac output with reduced systemic perfusion
occurs.
References
1. Jenkins NP, Ward C. Coarctation of the aorta: natural history
and outcome after surgical treatment. Q J Med 1999;92:365–
71.
2. Lim DS, Ralston MA. Echocardiographic indices of Doppler
flow patterns compared with MRI or angiographic measure-
ments to detect significant coarctation of the aorta. Echocar-
diography 2002;19:55–60.
3. Rothman A. Coarctation of the aorta, an update. Curr Probl
Pediatr 1998;28:37–60.
4. Grech V. Diagnostic and surgical trends, and epidemiology
of coarctation of the aorta in a population-based study. Int
J Cardiol 1999;68:197–202.
5. Strattford MA, Griffiths SP, Gersony WM. Coarctation of the
aorta, a study in delayed detection. Pediatrics 1982;69:159–
63.
6. Thoele DG, Master AJ, Paul MH. Recognition of the coarc-
tation of the aorta: a continuing challenge for the primary
care physician. Am J Dis Child 1987;141:1201–4.
7. Robinson PJ, Wyse RKH, Deanfield JE, et al. Continues wave
doppler velocimetry as an diagnosis of critical left heart
obstruction in neonates. Br Heart J 1984;52:552–6.
8. Rinelli G, Marino B, Santoro G, et al. Pitfalls in echocardio-
graphic-based repair of aortic coarctation. Am J Cardiol
1997;80:1382–3.
9. Stern HC, Locher D, Wallnofer K, et al. Noninvasive assess-
ment of coarctation of the aorta: comparative measurements
by two-dimensional echocardiography, magnetic resonance,
and angiography. Pediatr Cardiol 1991;12:1–5.
10. Muhler EG, Neuerburg JM, Ruben A, et al. Evaluation of
aortic coarctation after surgical repair: role of magnetic
resonance imaging and Doppler ultrasound. Br Heart J
1993;70:285–90.
11. Seifert BL, DesRochers K, Ta M, et al. Accuracy of Doppler
methods for estimating peak-to-peak instantaneous gradi-
ents across coarctation of the aorta: an in vitro study. J Am
Soc Echocardiogr 1999;12:744–53.
12. Morrow WH, Huhta JC, Murphy DJ, et al. Quantitative
morphology of the aortic arch in neonatal coarctation. J Am
Coll Cardiol 1986;8:616–20.
13. Aluquin VPR, Shutte D, Nihill MR, et al. Normal aortic arch
growth and comparison with isolated coarctation of the
aorta. Am J Cardiol 2003;91:502–5.
14. Nihoyannopoulos P, Karas S, Sapsford RN, et al. Accuracy of
two-dimensional echocardiography in the diagnosis of aortic
arch obstruction. J Am Coll Cardiol 1987;10:1072–7.
15. Lee EY, Siegel MJ, Hildebolt CF, Gutierrez FR, Bhalla S,
Fallah JH. MDCT evaluation of thoracic aortic anomalies in
pediatric patients and young adults: comparison of axial,
multiplanar, and 3D images. AJR Am J Roentgenol 2004;182:
777–84.
INVITED COMMENTARY
This article [1] describes a novel and potentially impor-
tant new echocardiographic index for the diagnosis of
coarctation of the aorta in neonates and infants. The
authors have proposed the index because of the frequent
difficulty in confidently establishing the diagnosis of
coarctation, particularly in the smallest and youngest
patients. Three anatomic features create this difficulty:
the coexistence of a large ductus arteriosus, the presence
of hypoplasia of the aortic arch, and the lack of “co-
planarity” of the aortic arch, ductus, and descending
aorta. Previous investigators [2, 3] have suggested that
specific dimensional thresholds for the aortic isthmus of
4.5 mm [2] or 3 mm [3] allow the diagnosis of coarctation.
However the specificity and sensitivity of such a measure
are far from perfect, and the application of either stan-
dard to very small infants will certainly lead to overdiag-
nosis of coarctation. The addition of Doppler assessments
has variously been believed to be of limited value [4] or of
significant help if combined with size criteria [3]. In
present day practice, despite the several proposed diag-
nostic tests for coarctation, it is still quite common to
allow the ductus to close under observation to allow a
coarctation to “declare itself” if present. Such a declara-
tion will take the form of the acute development of aortic
obstruction with potential consequences of distal hypo-
perfusion and metabolic acidosis, renal injury, left ven-
tricular dysfunction, pulmonary edema, and pulmonary
hypertension. In effect, the patient is forced to prove he
has a disease by becoming ill.
The validation of the carotid-subclavian artery index
would allow the relegation of observed ductal closure to
the slagheap of history where it rightly belongs. The
measurements required to calculate the index are readily
obtained from standard suprasternal views of the distal
arch. Accurately aligned Doppler windows are not re-
quired, and there is no necessity for co-planarity of the
aortic arch, ductus, and descending aorta. There is also
no requirement for detecting a “coarctation shelf” as
described by other authors [5]. Another advantage of
using the index is the fact that it is a ratio, and thus it
would not be confounded by extremely small patient
size.
However several caveats are worth mentioning in
regard to the new measure, which has not yet been tested
in other centers. Despite the excellent sensitivity and
specificity of this index, it is important that it not be
applied in isolation. There is the occasional neonate, with
transverse aortic arch hypoplasia and a large patent
ductus arteriosus, who does not develop coarctation of
the aorta, and an aggressive strategy of surgical interven-
tion in these patients based on an as-yet unconfirmed
echocardiographic index that could result in unnecessary
procedures and exposure to potential late complications,
such as recurrent arch obstruction and distortion. Beyond
1657Ann Thorac Surg DODGE-KHATAMI ET AL
2005;80:1652–8 CAROTID-SUBCLAVIAN ARTERY INDEX
© 2005 by The Society of Thoracic Surgeons 0003-4975/05/$30.00
Published by Elsevier Inc doi:10.1016/j.athoracsur.2005.07.011
CARDIOVASCULAR](https://image.slidesharecdn.com/a1371900-ea85-4211-a52f-11ae4a80a842-161219234510/75/CSI-6-2048.jpg)