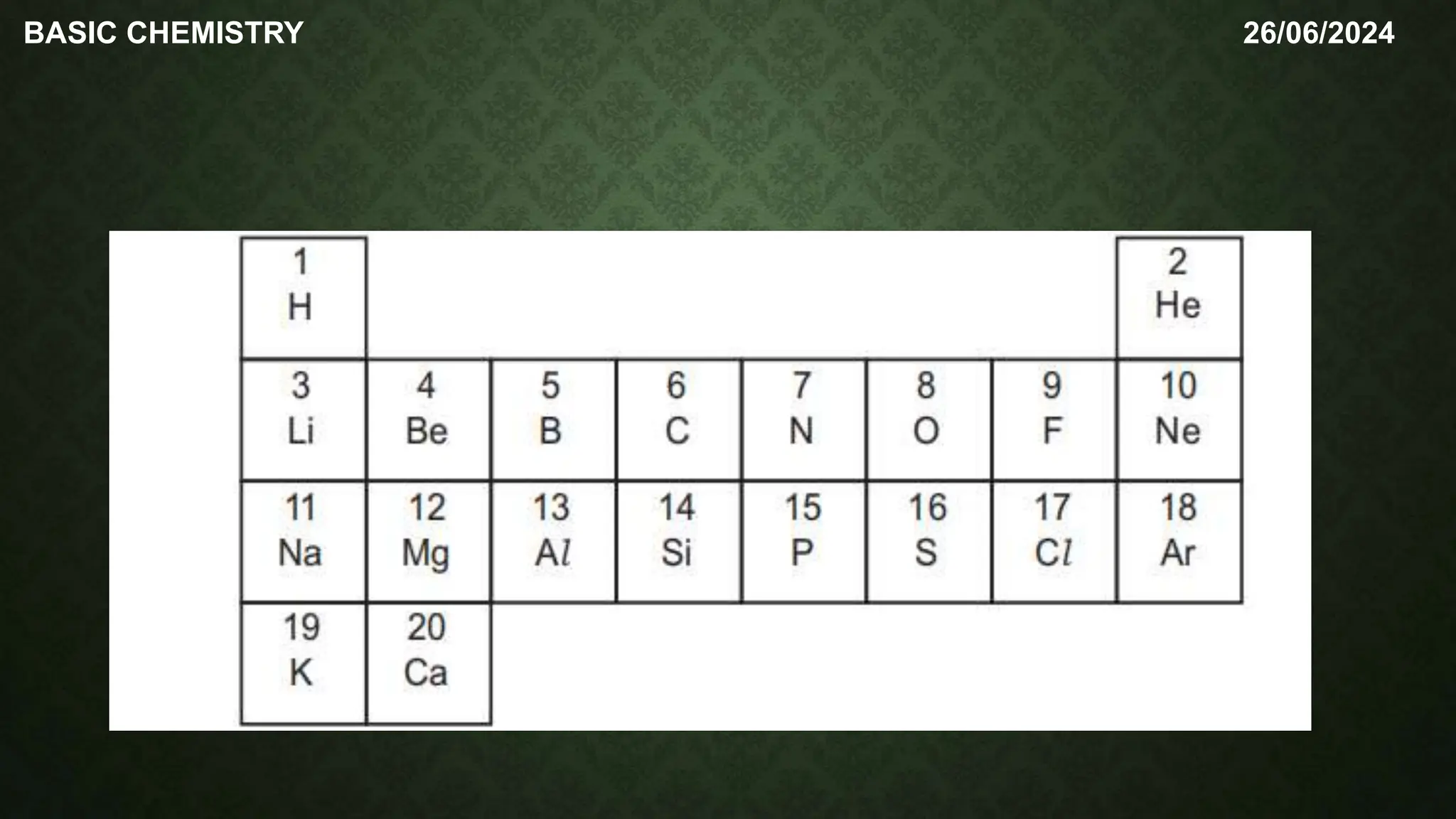
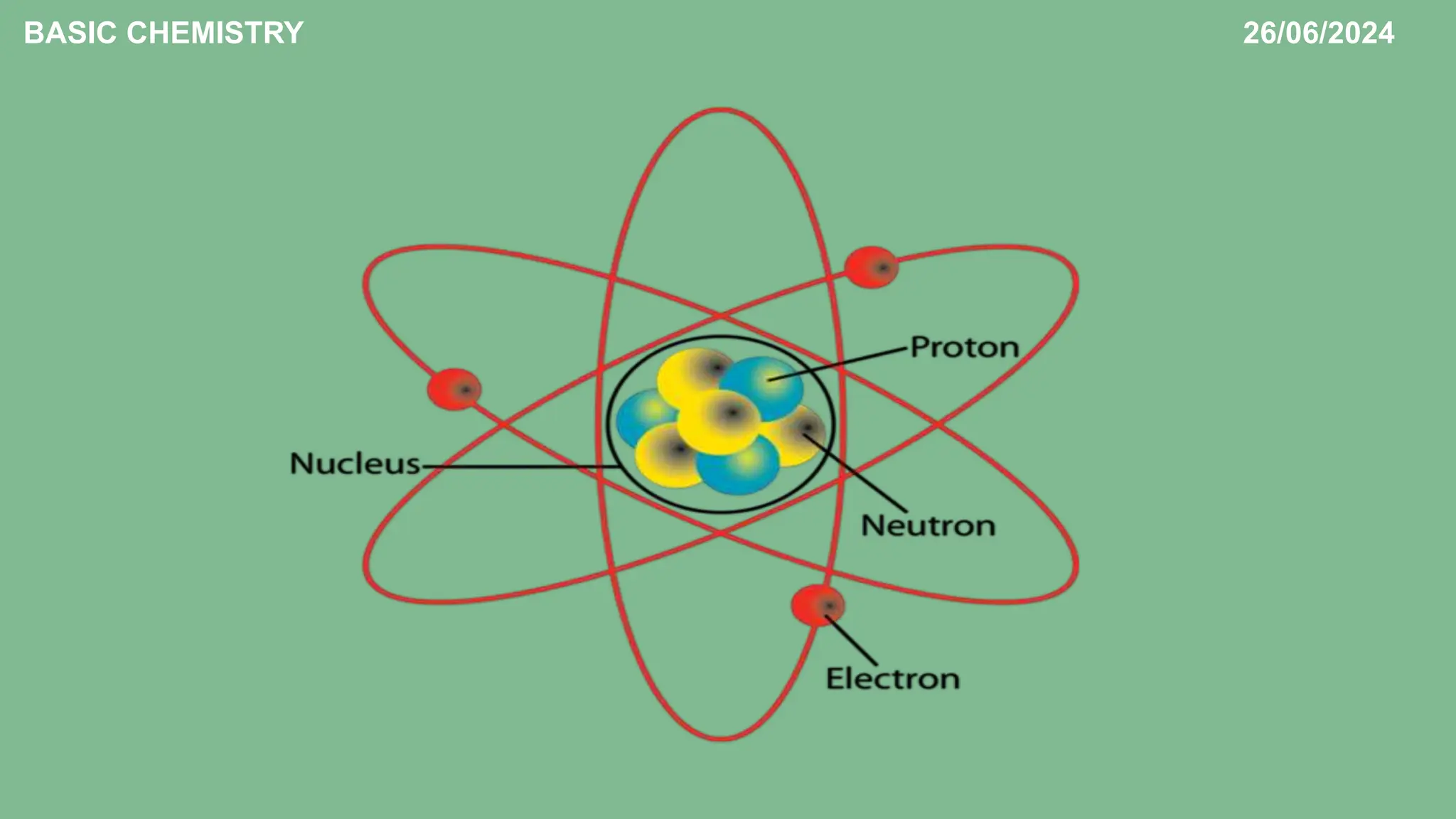
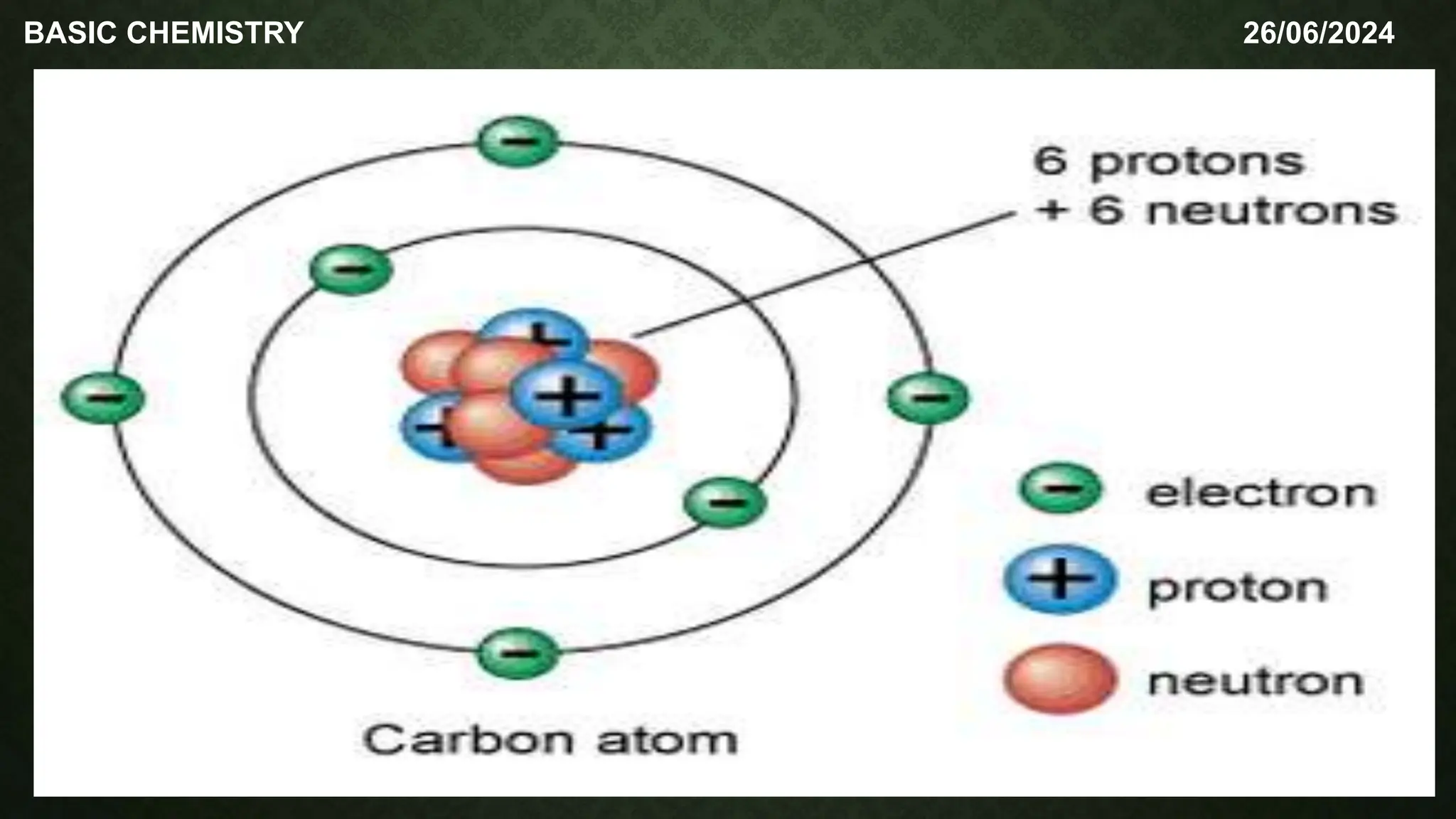
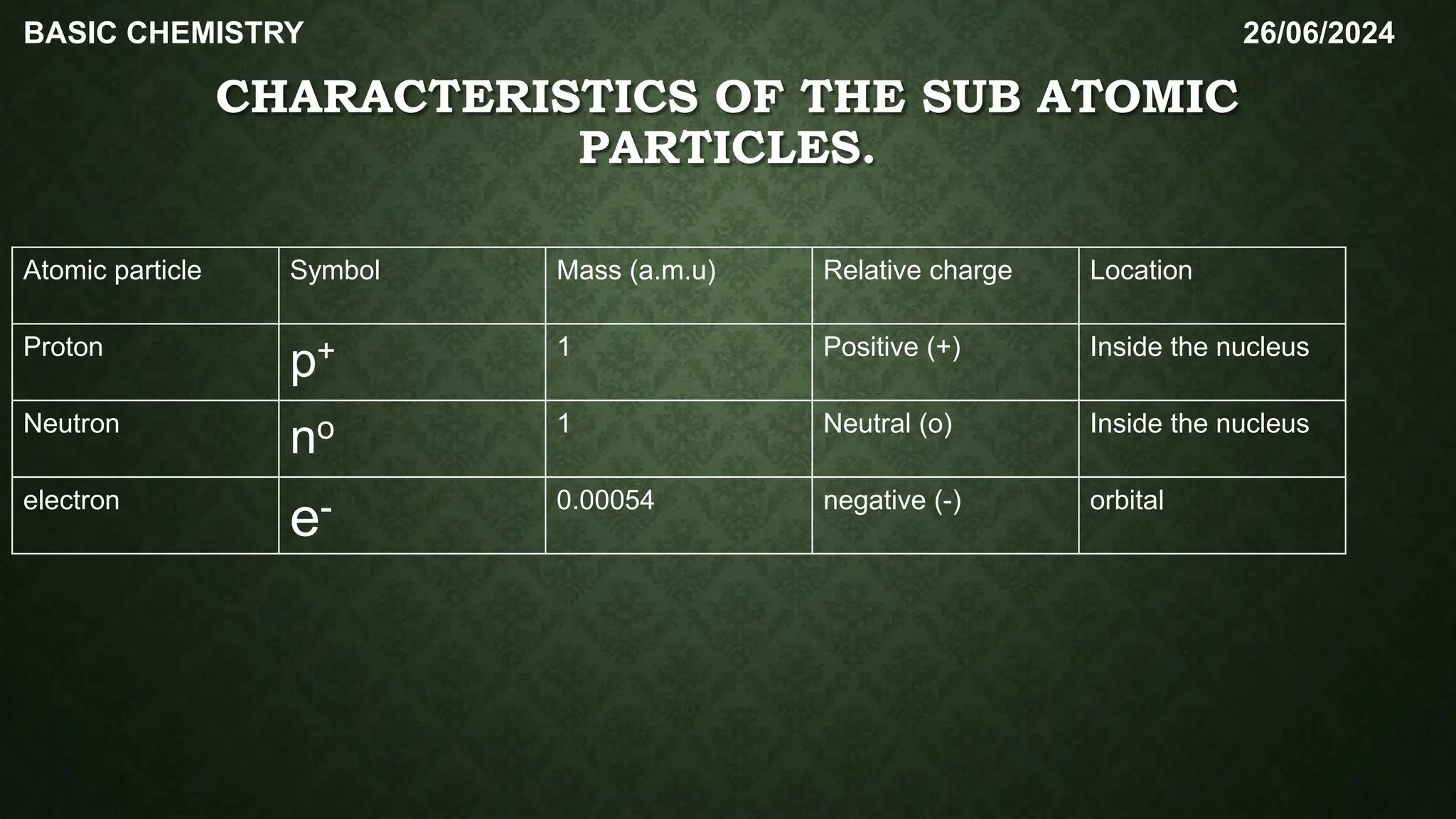
The document outlines the basic concepts of subatomic particles, including protons, neutrons, and electrons, detailing their structures, locations, charges, and masses. It highlights that while Dalton's atomic theory suggested atoms were indivisible, modern understanding recognizes that atoms can be broken into smaller subunits. Additionally, the document includes a summary of the contributions of scientists like Ernest Rutherford, James Chadwick, and J.J. Thompson in the discovery of these particles.