Embed presentation

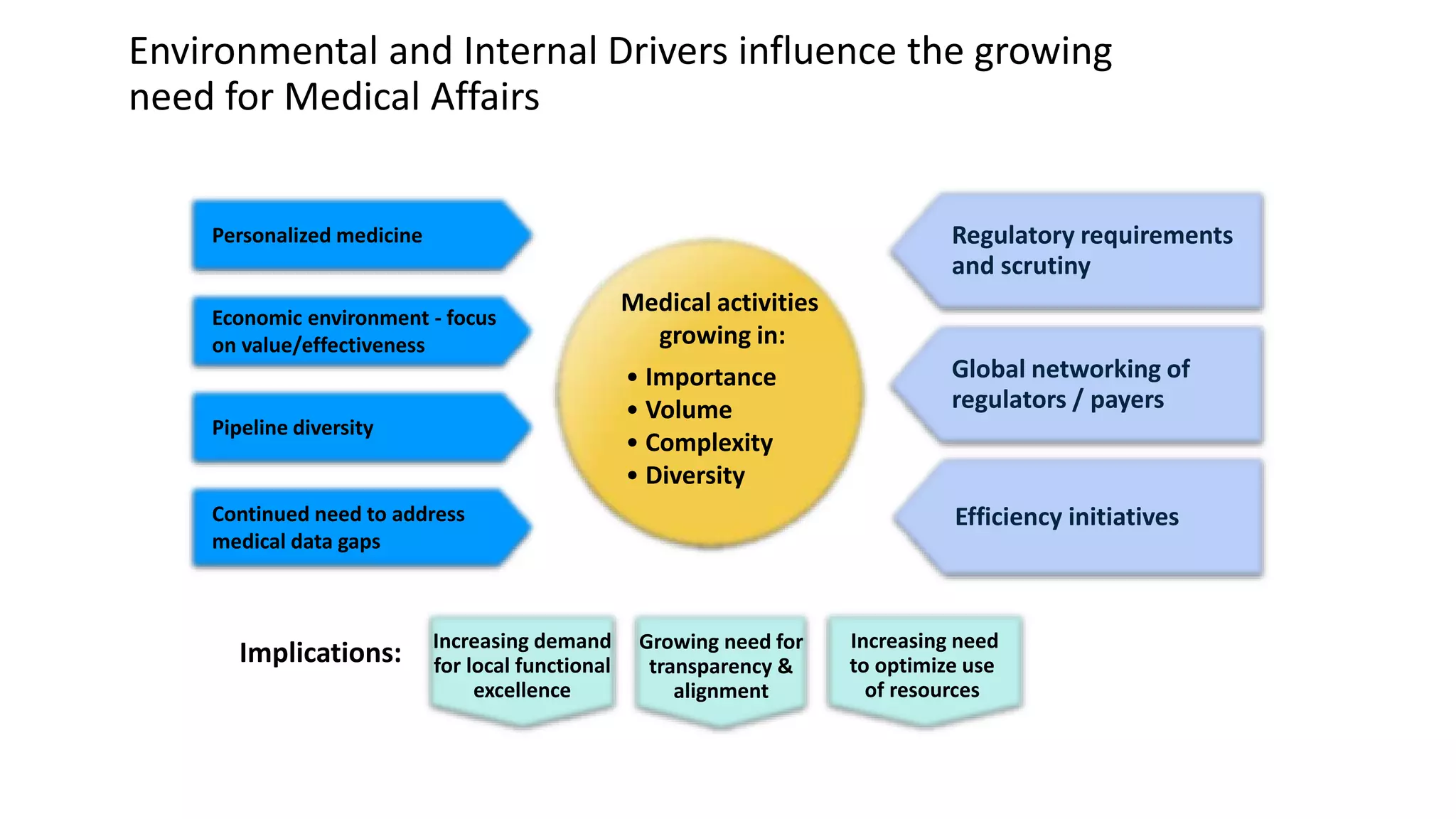
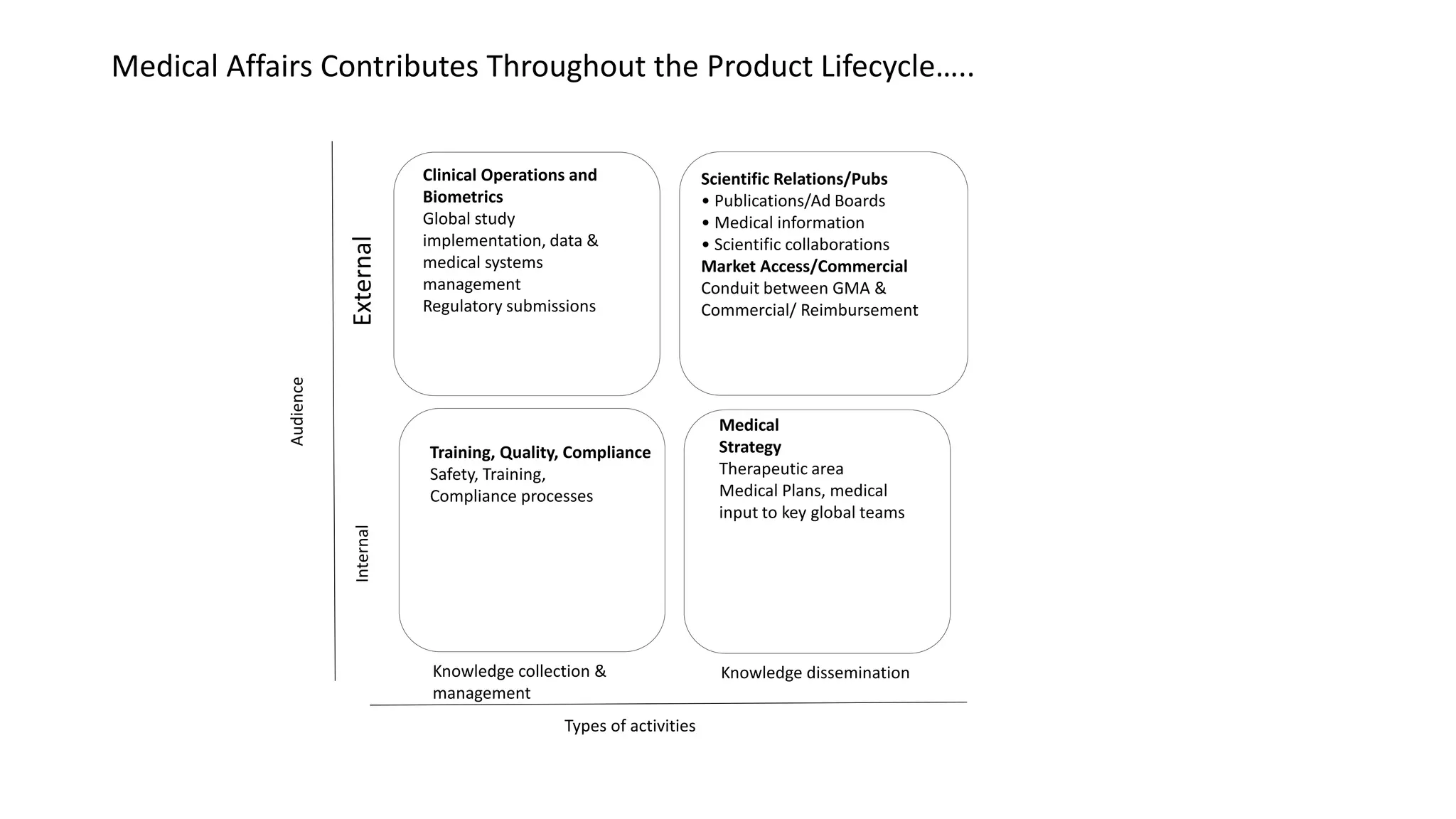
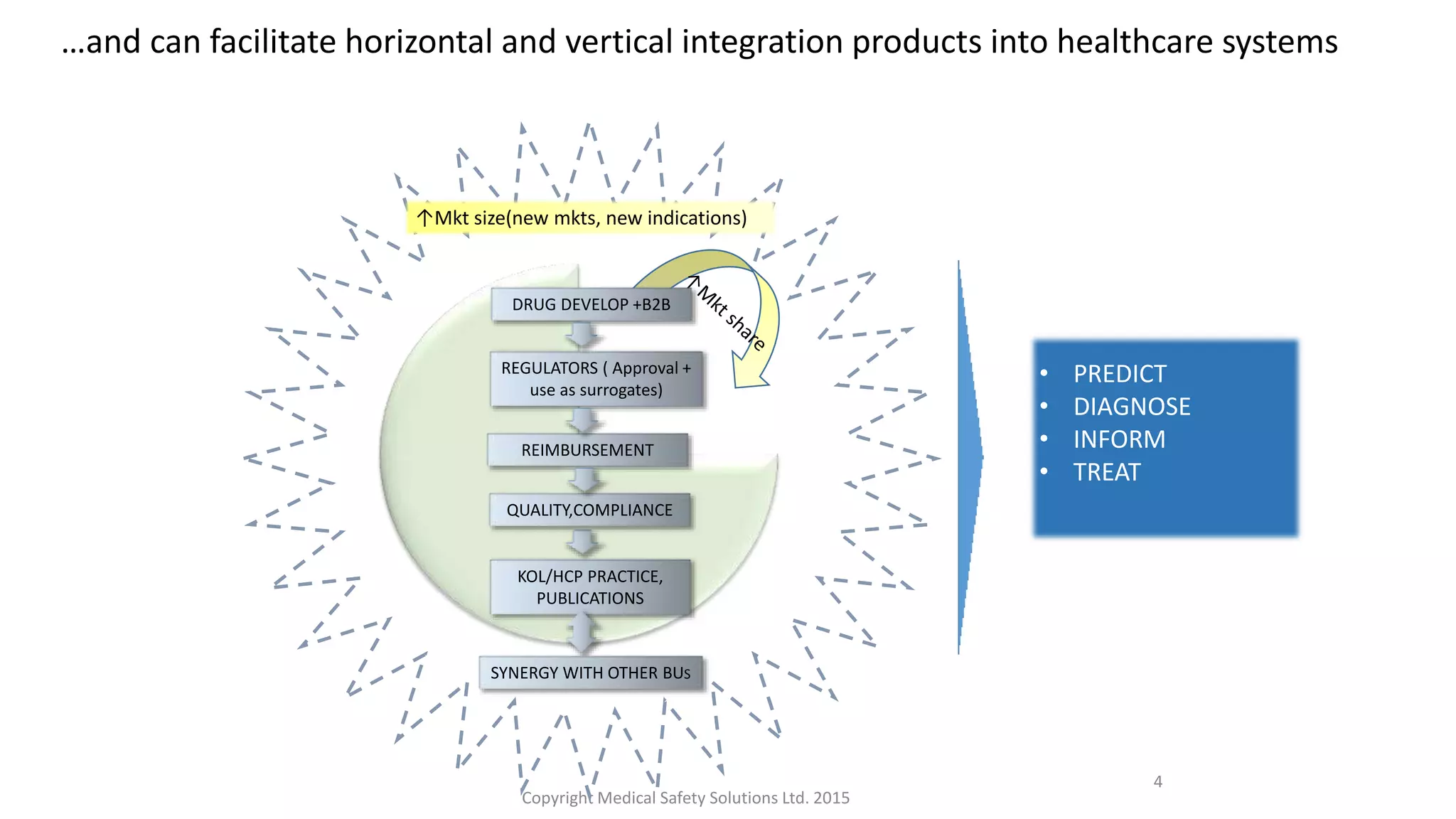
Strategic medical affairs in pharma and medical devices outlines key drivers influencing the growing role of medical affairs functions. Regulatory requirements, demand for local excellence, and the need for transparency are pushing medical activities to expand in areas like medical strategy, clinical operations, and regulatory submissions. Medical affairs contributes throughout the product lifecycle by collecting and disseminating knowledge to internal and external audiences, and can help integrate products into healthcare systems from drug development to reimbursement.



