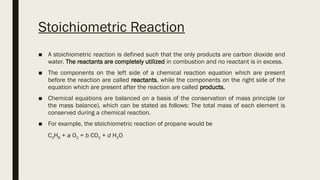
The document discusses the principles of combustion, defining it as a chemical reaction where fuel combines with oxygen to release energy. It outlines different types of combustion reactions, including stoichiometric, lean, and rich mixtures, along with their balancing through stoichiometry. Additionally, it describes the impact of air composition on combustion efficiency and the significance of the fuel/air equivalence ratio in engine performance.