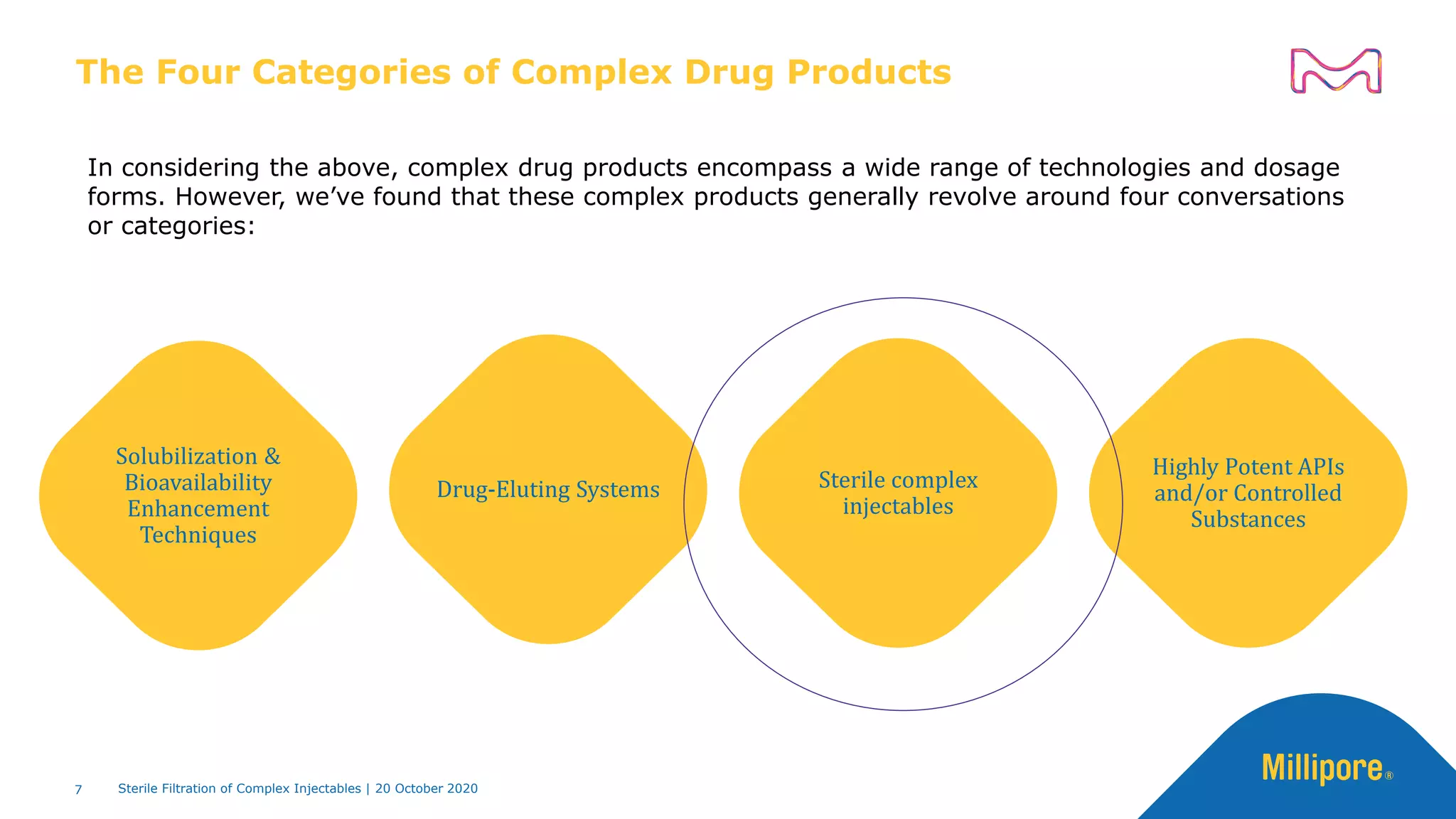
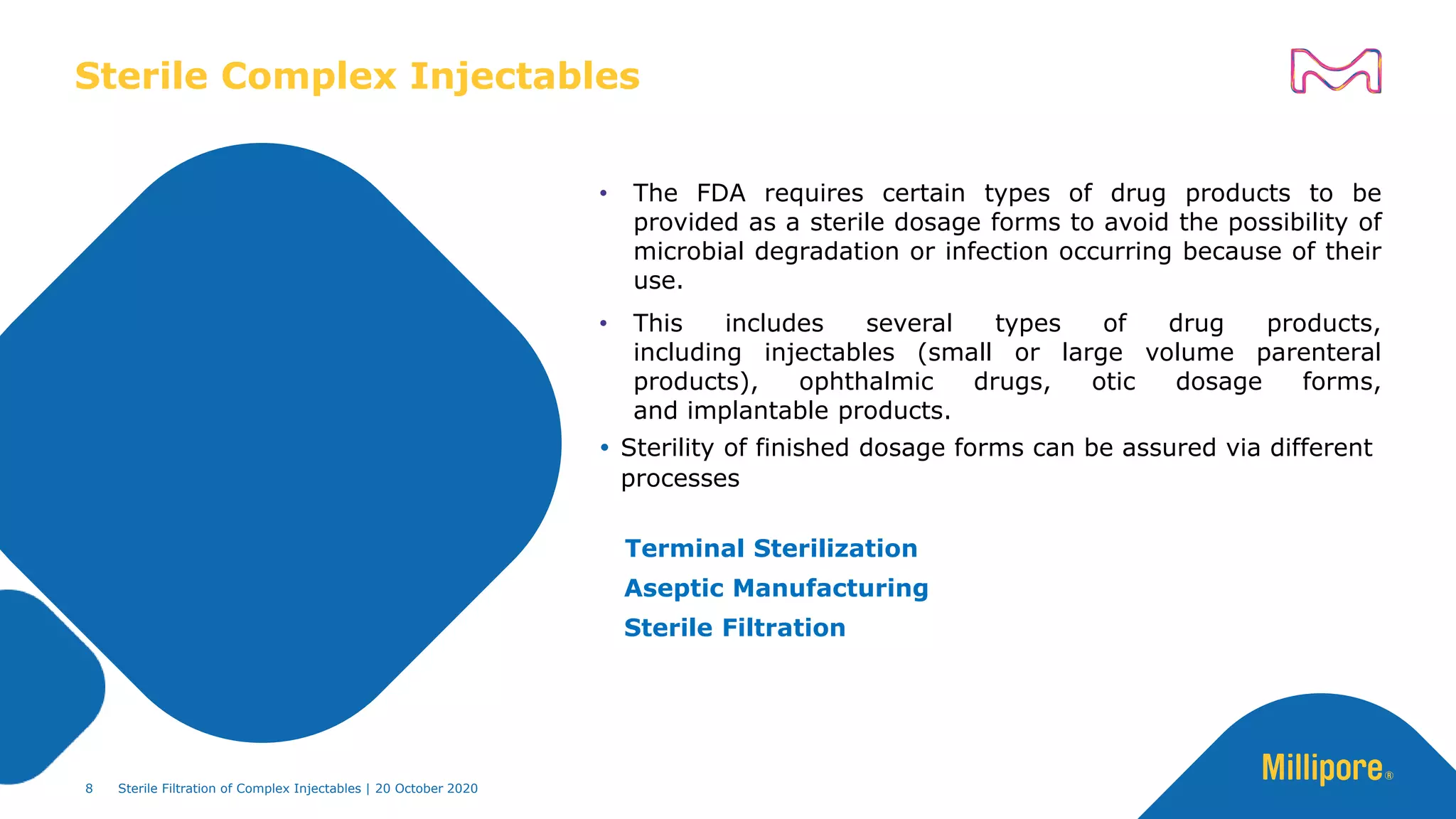
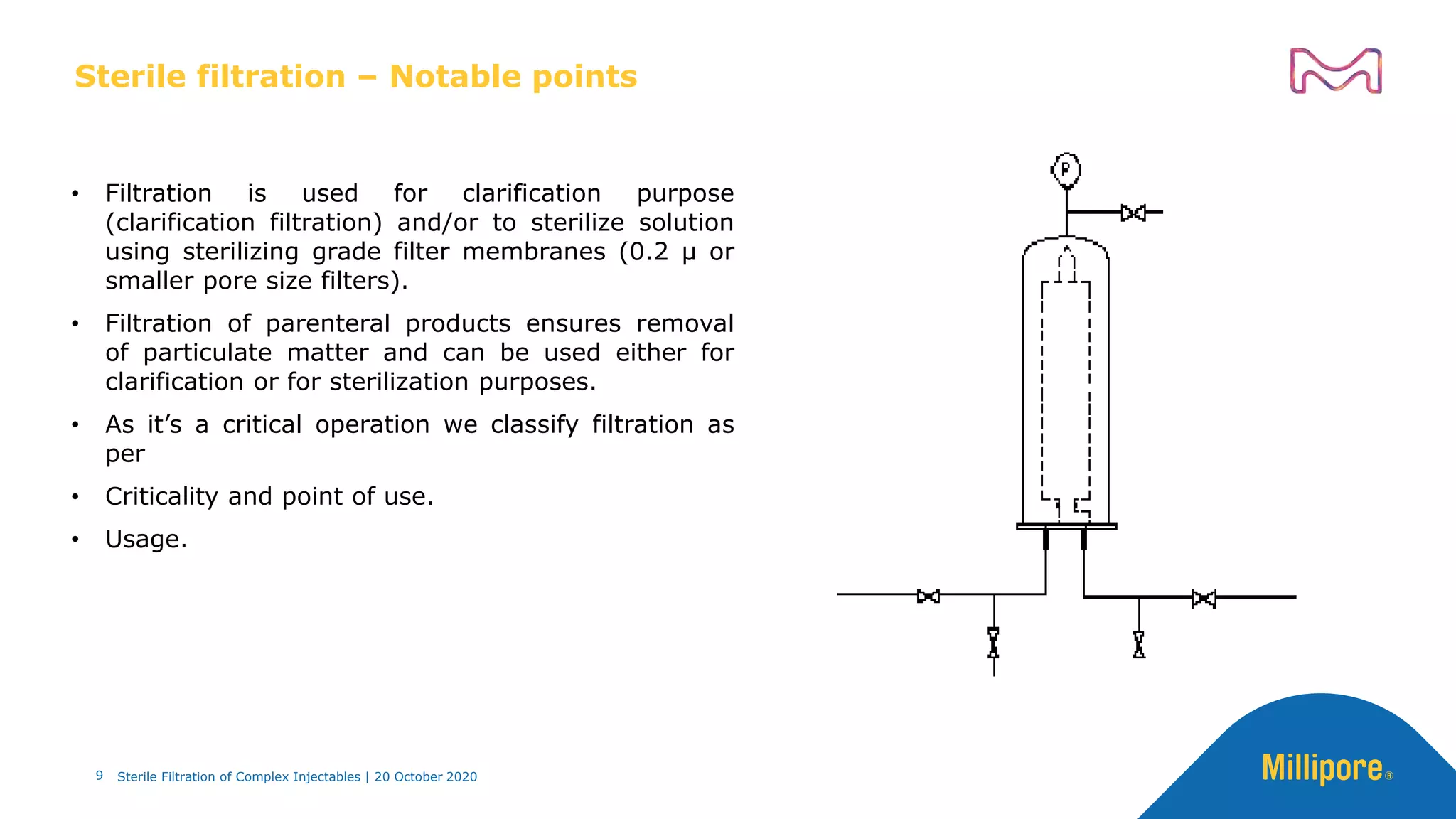
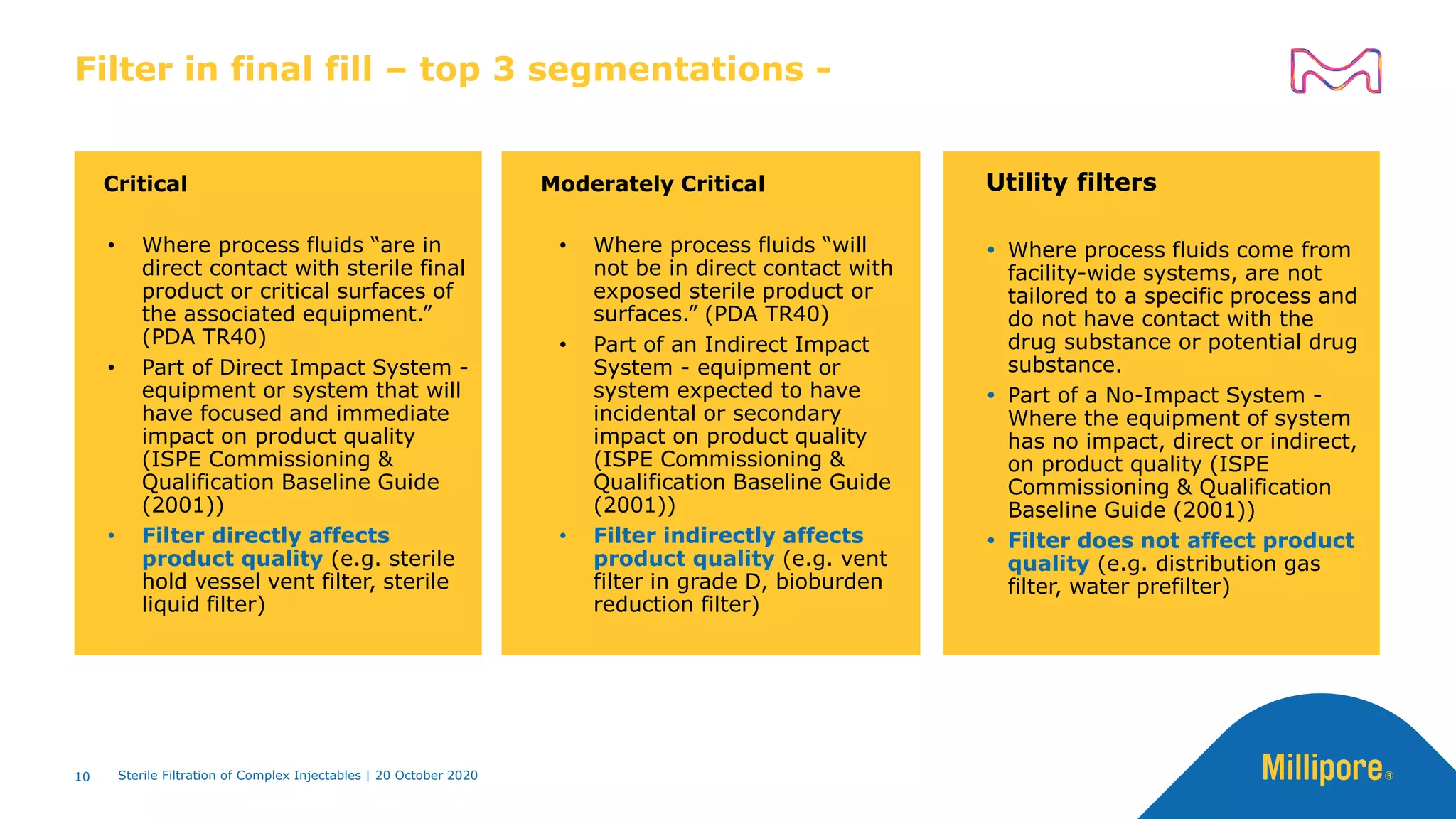
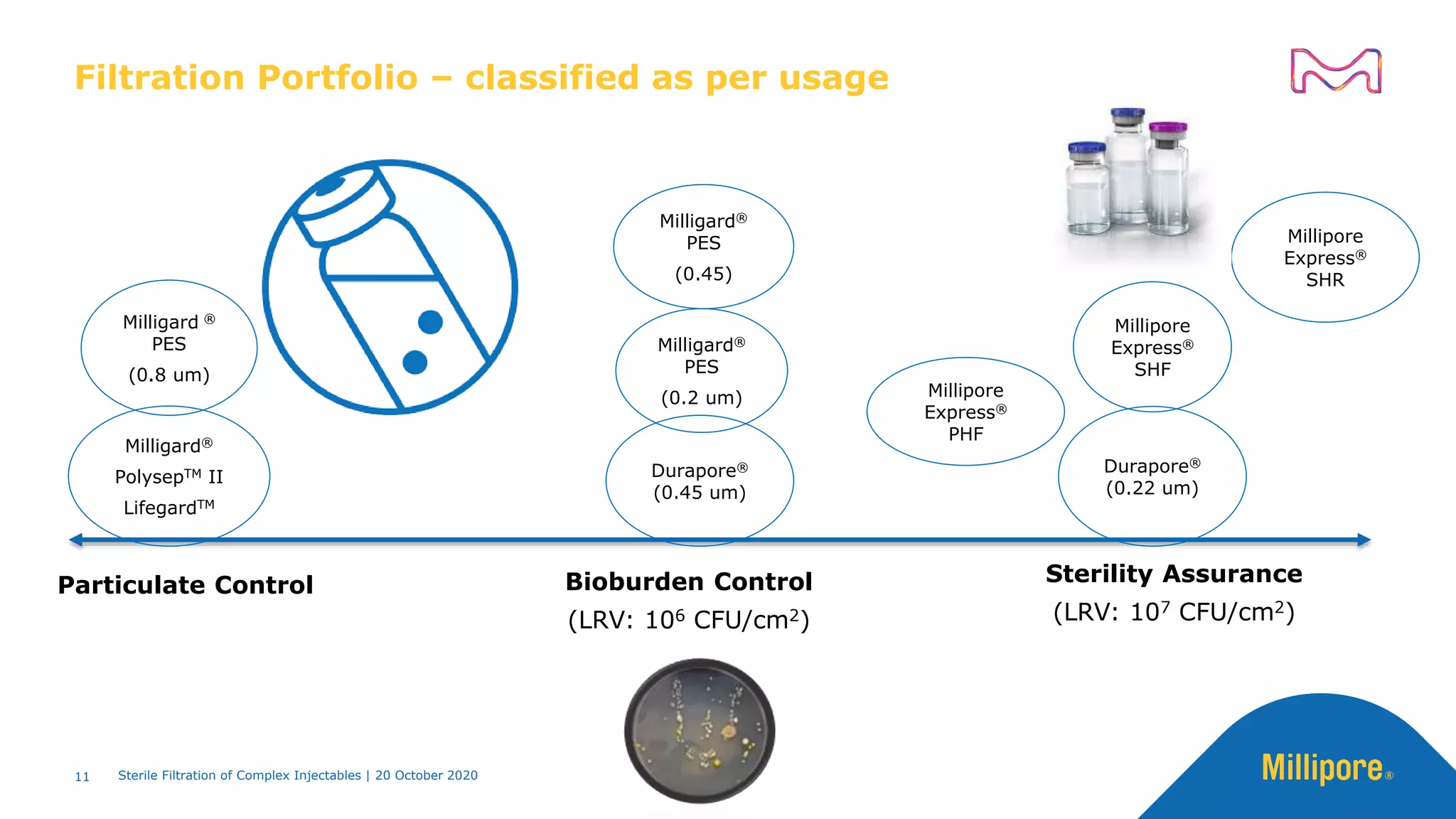
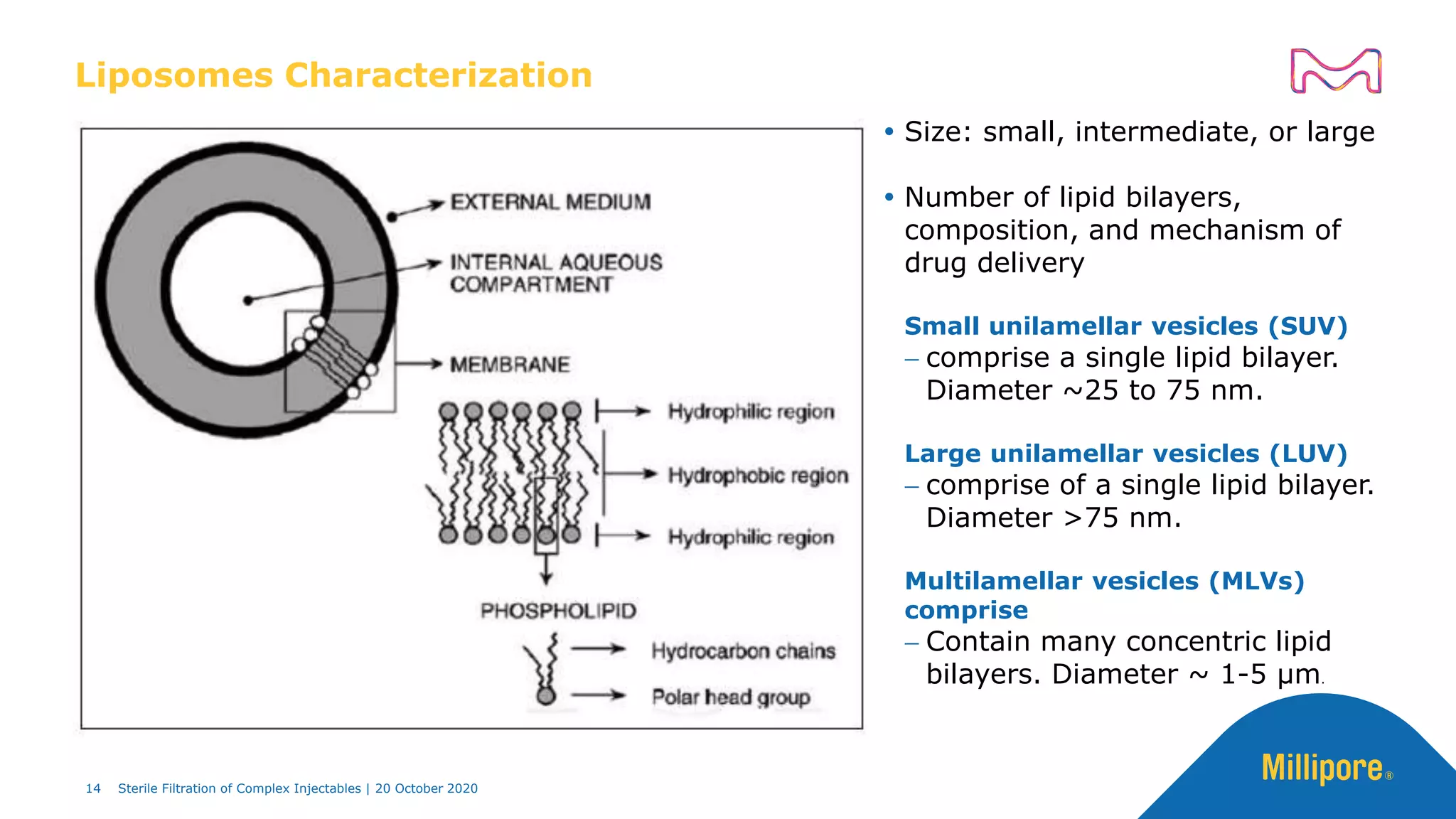
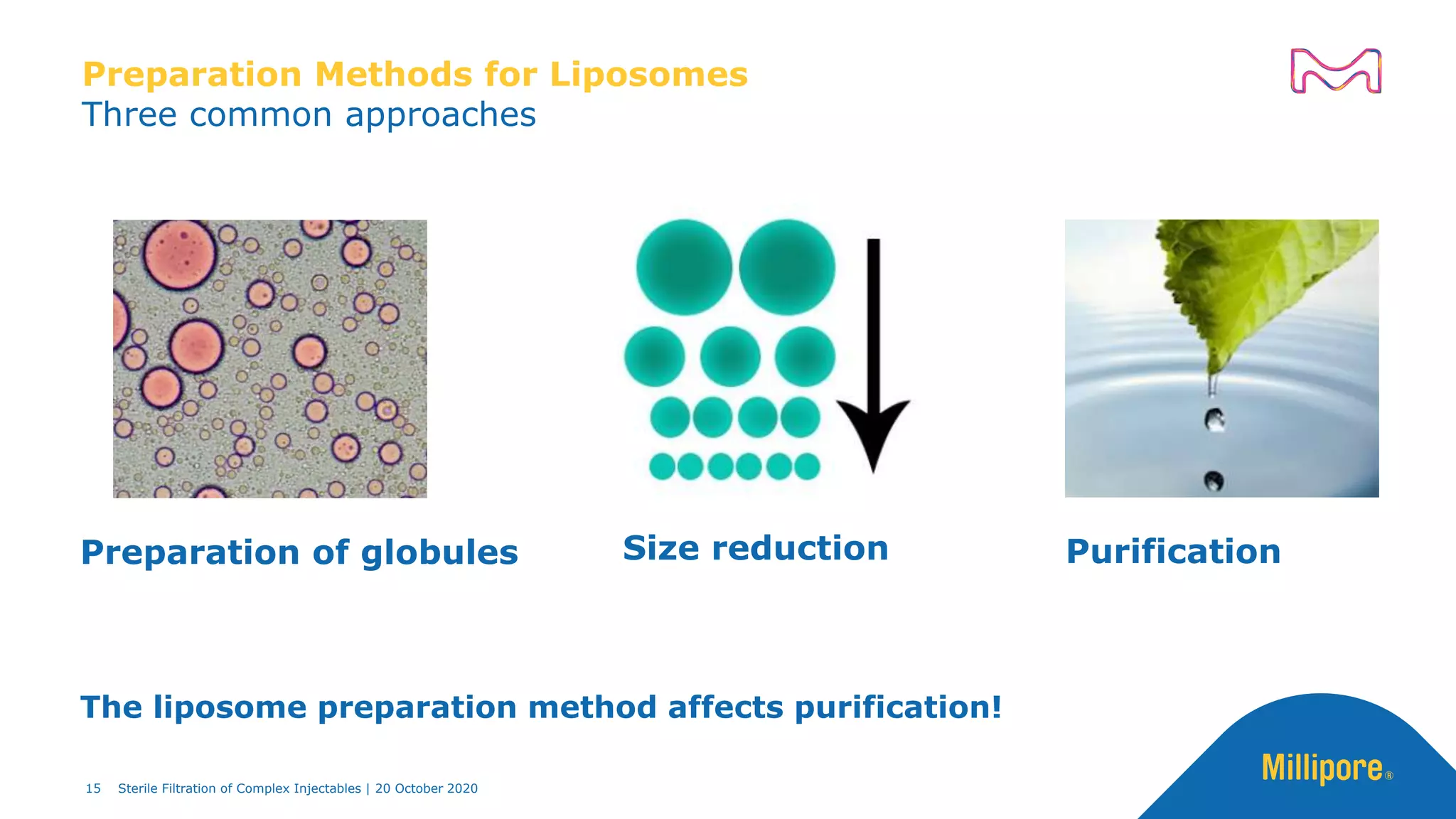
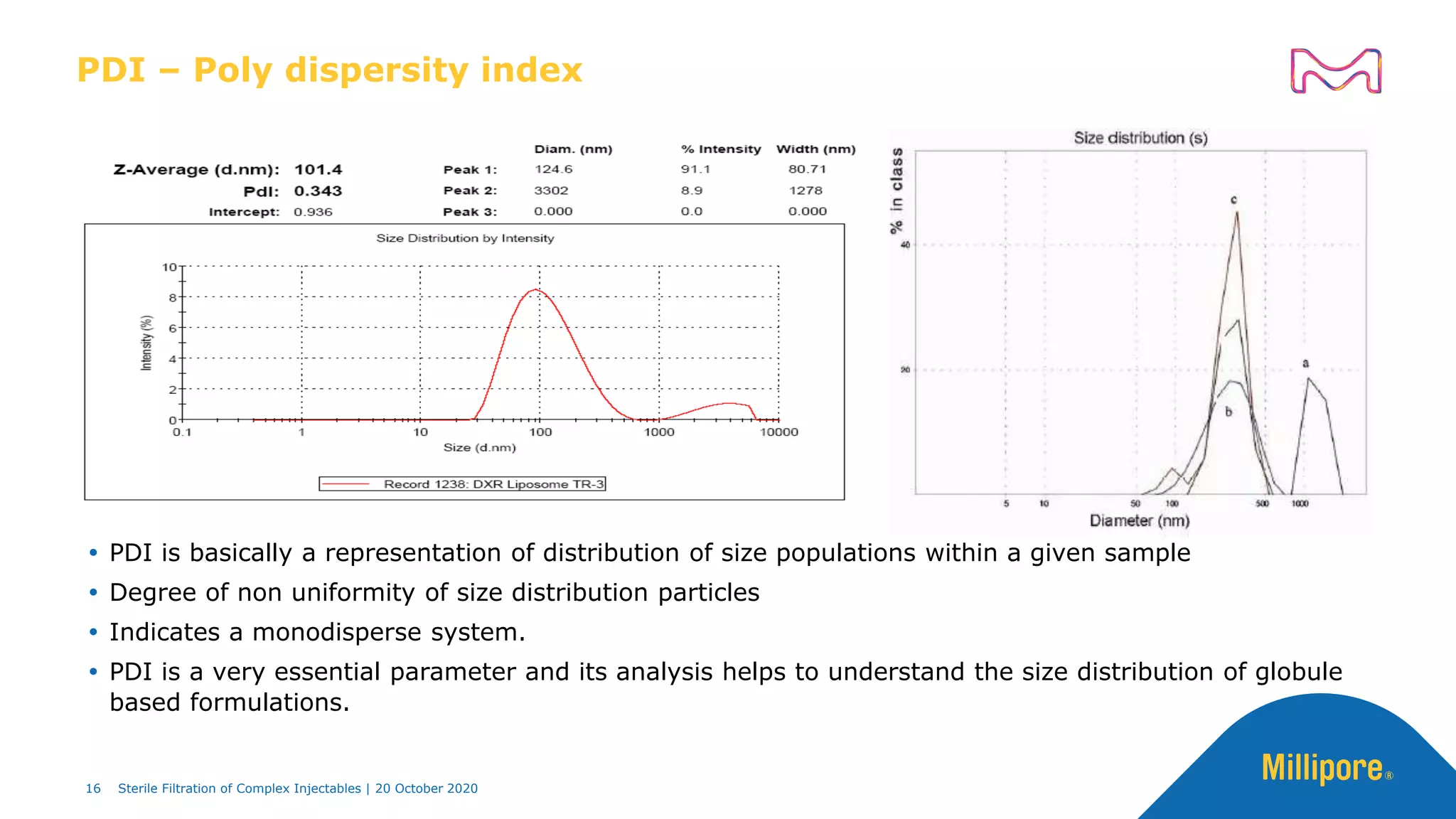
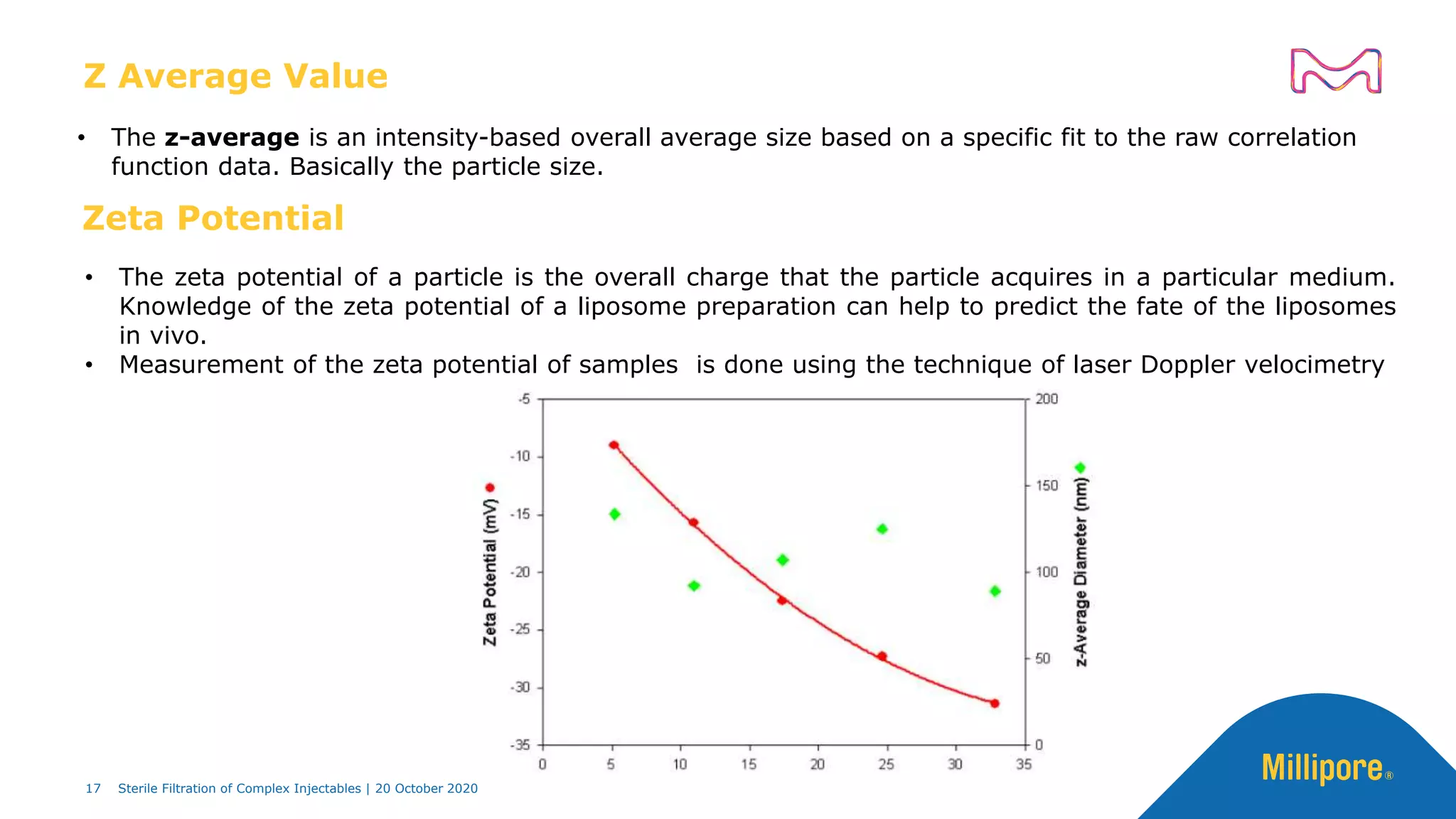
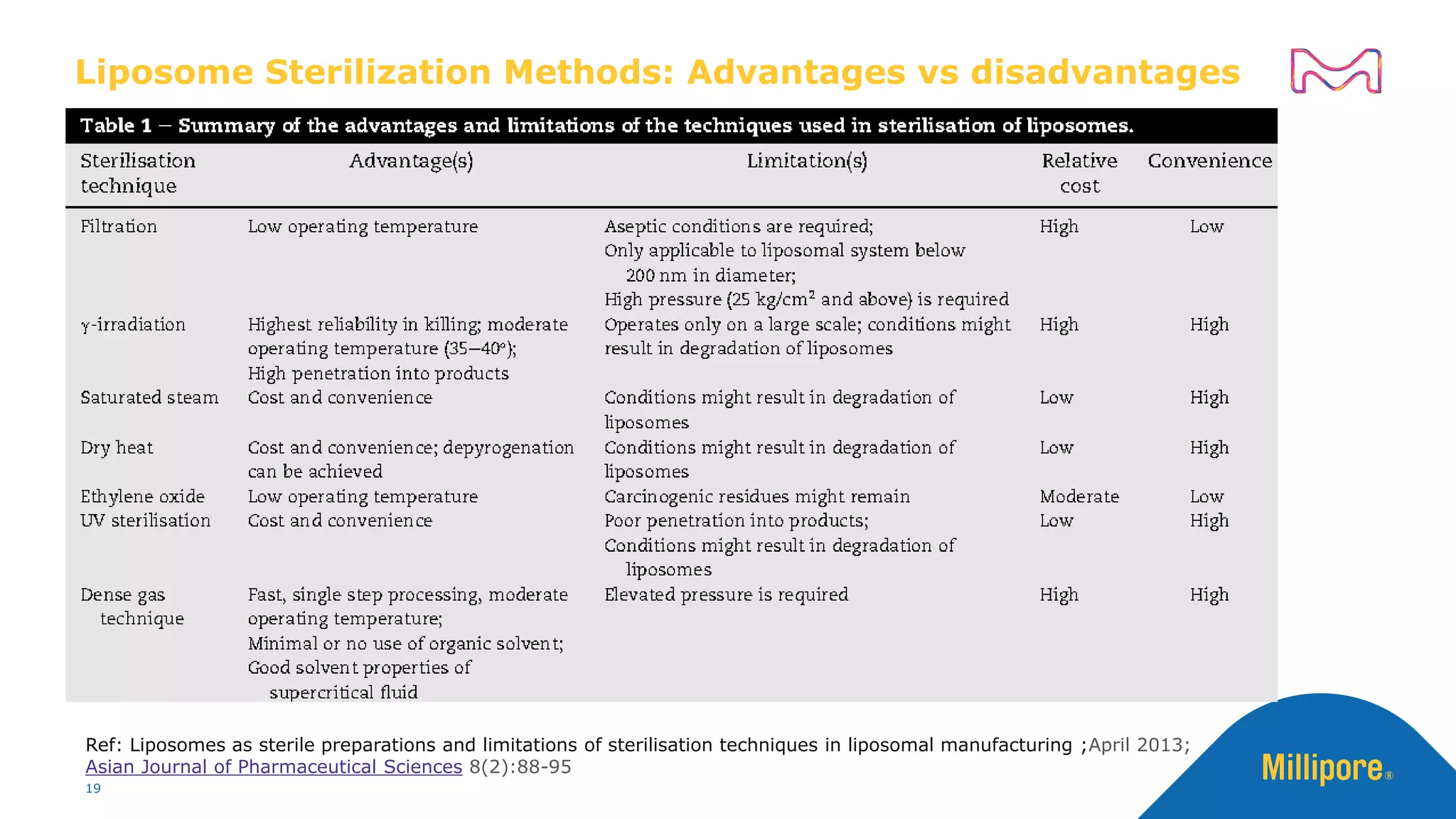
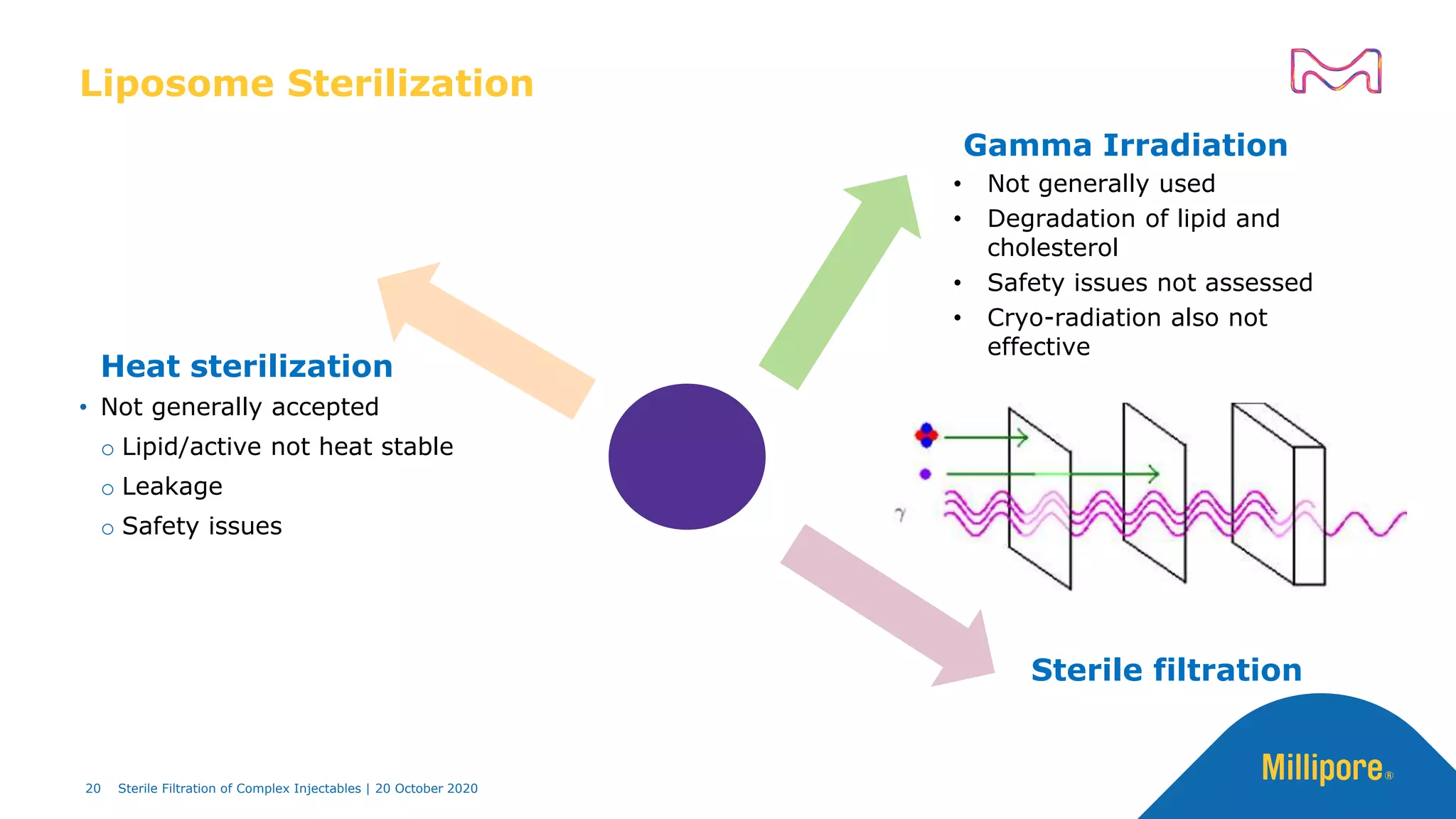
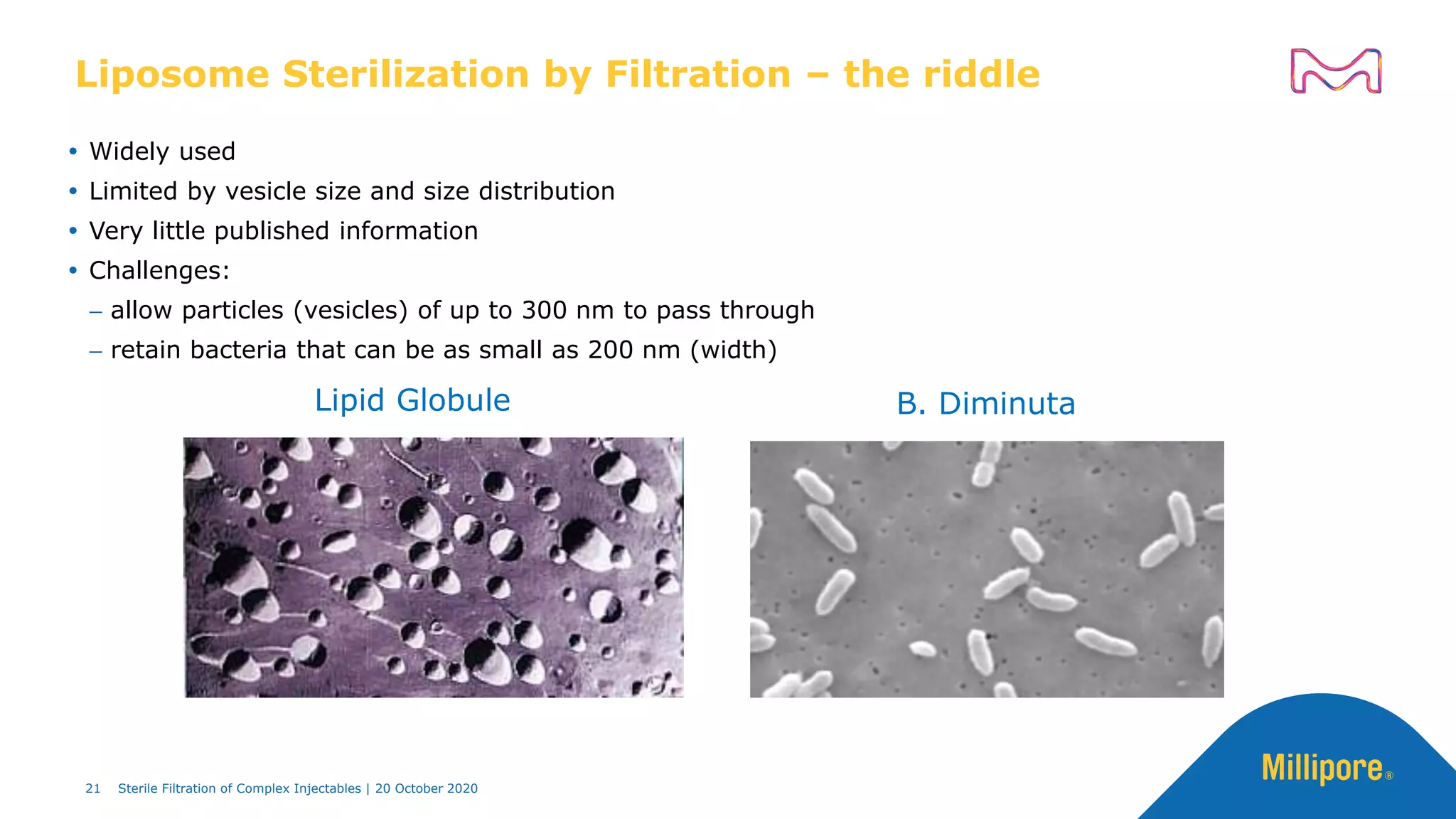
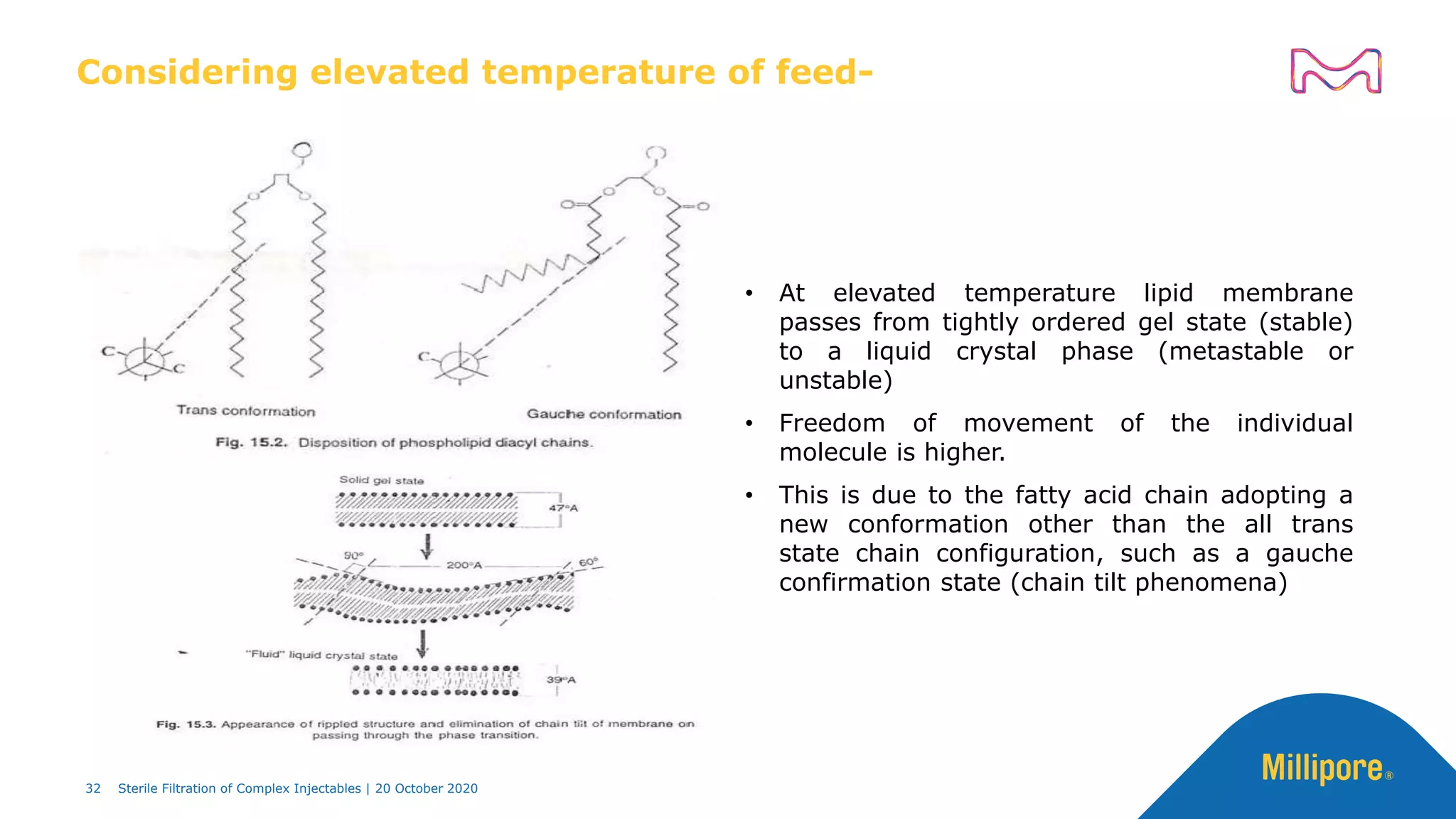
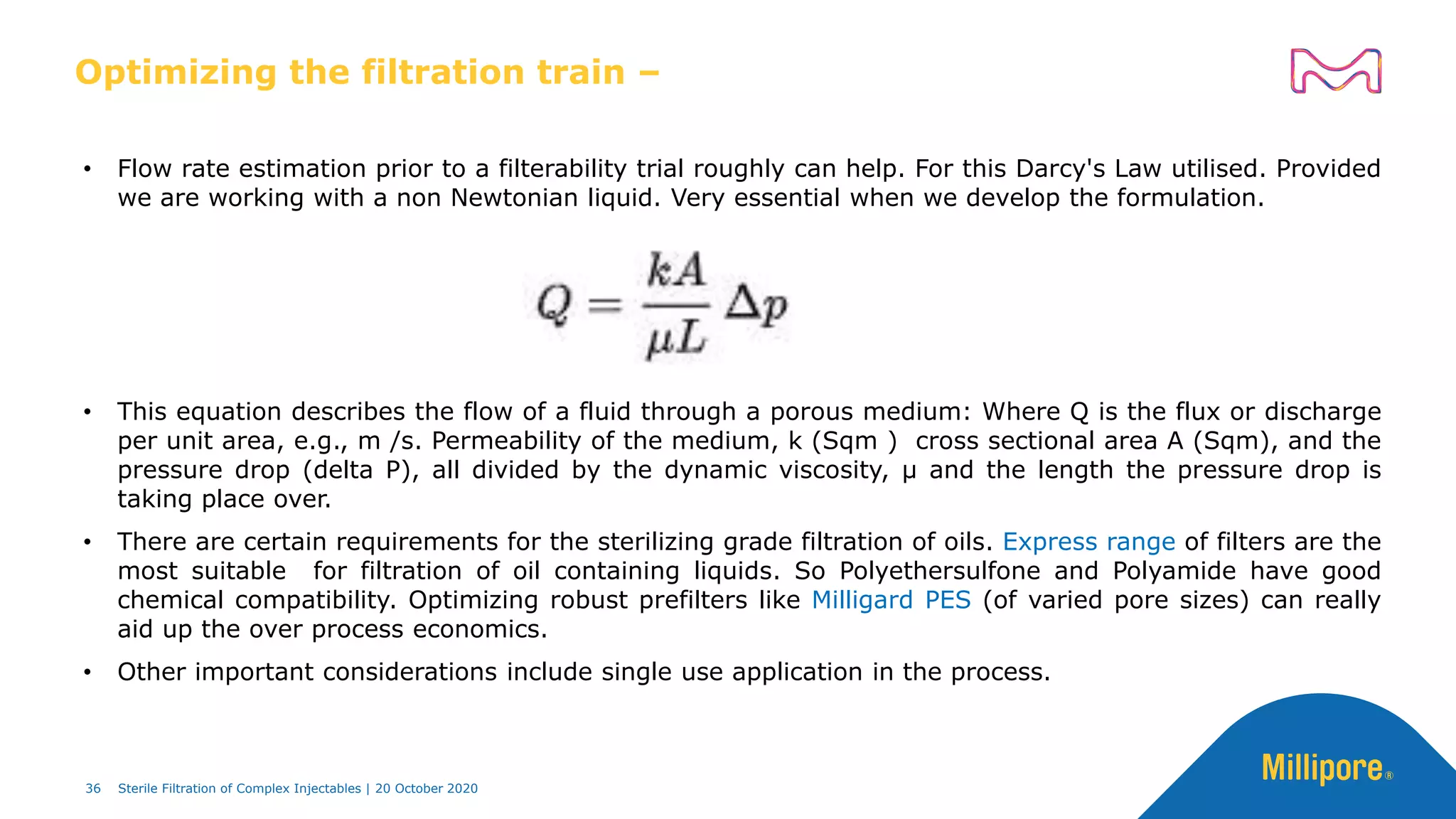
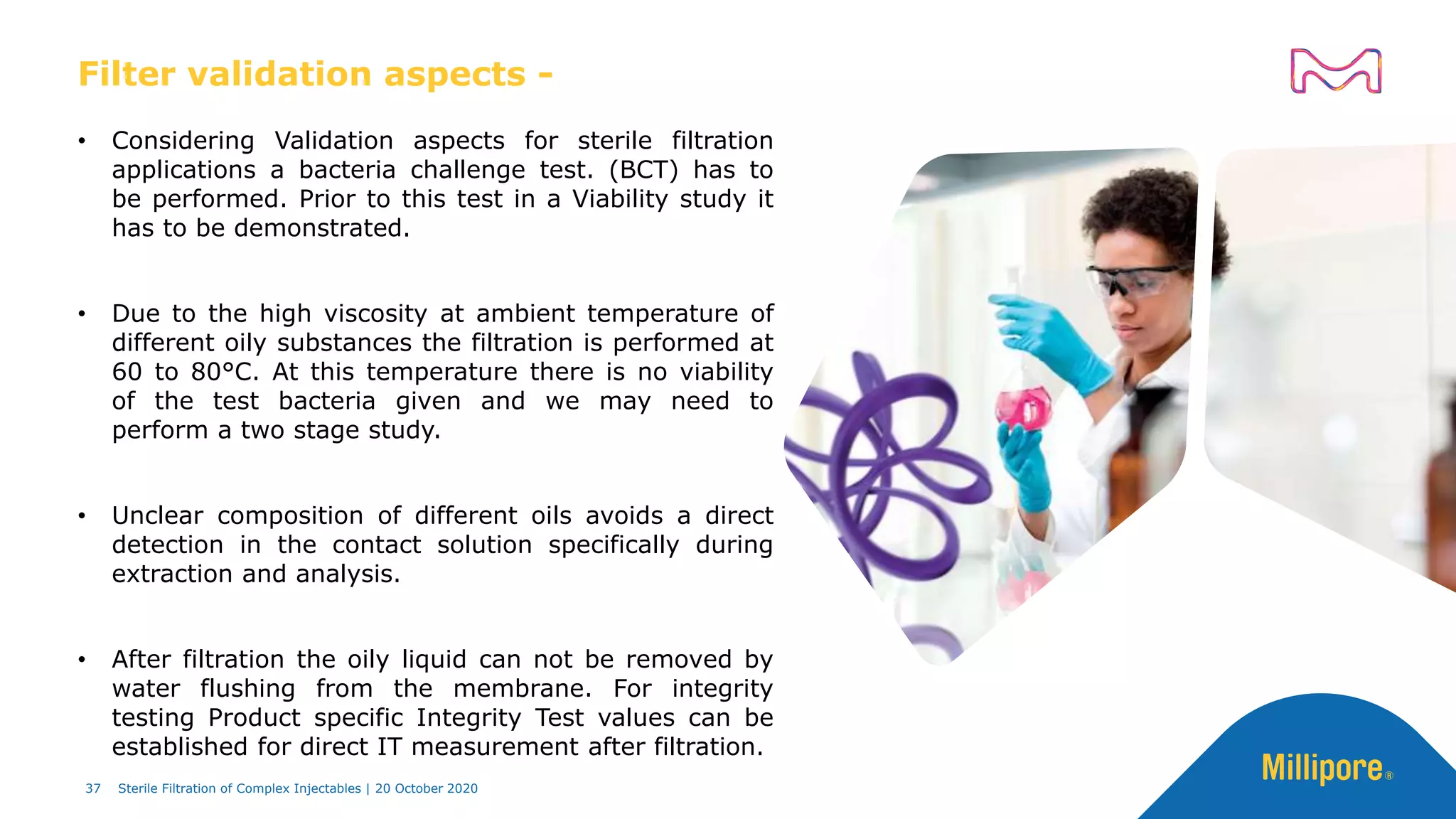
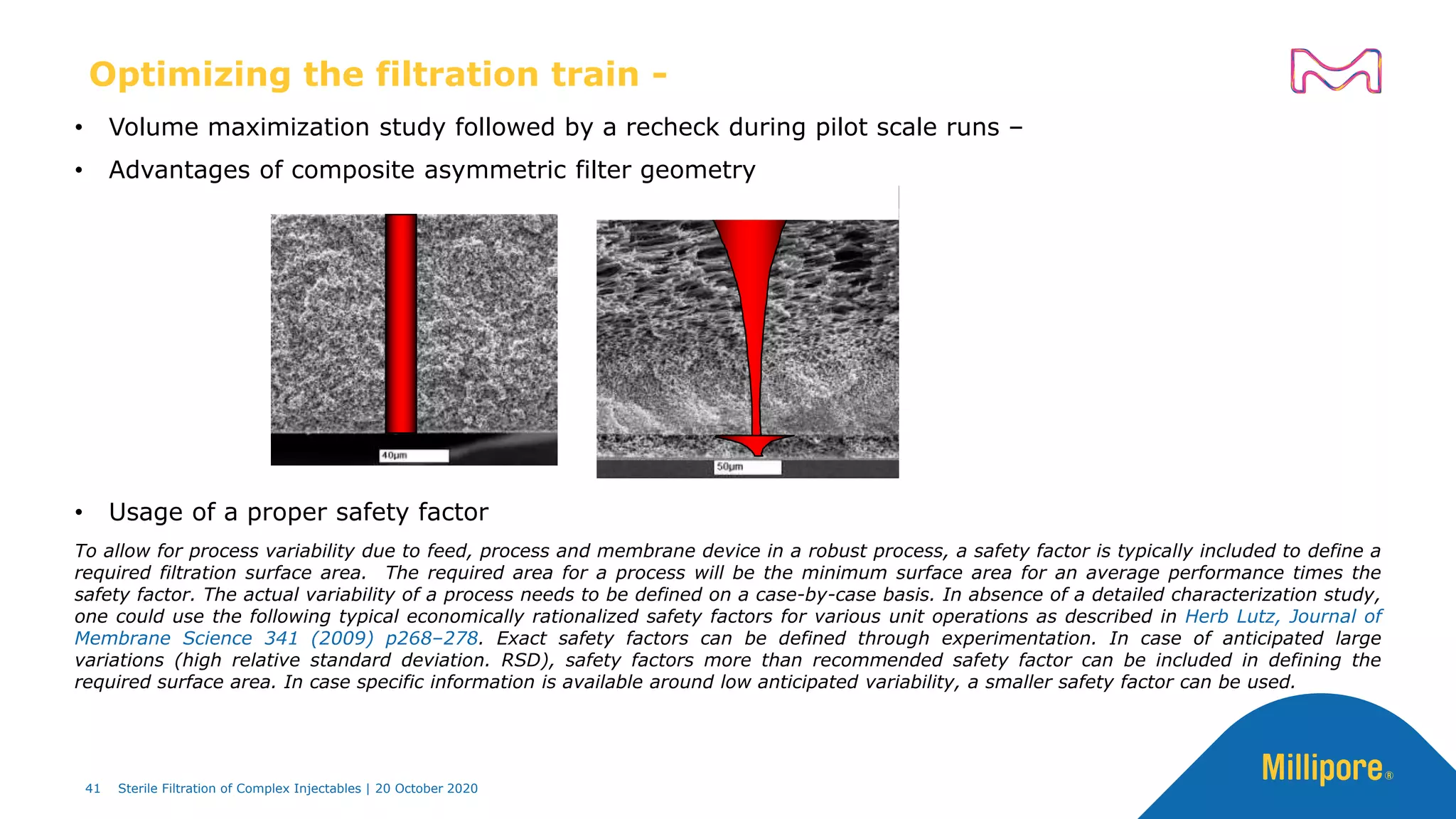
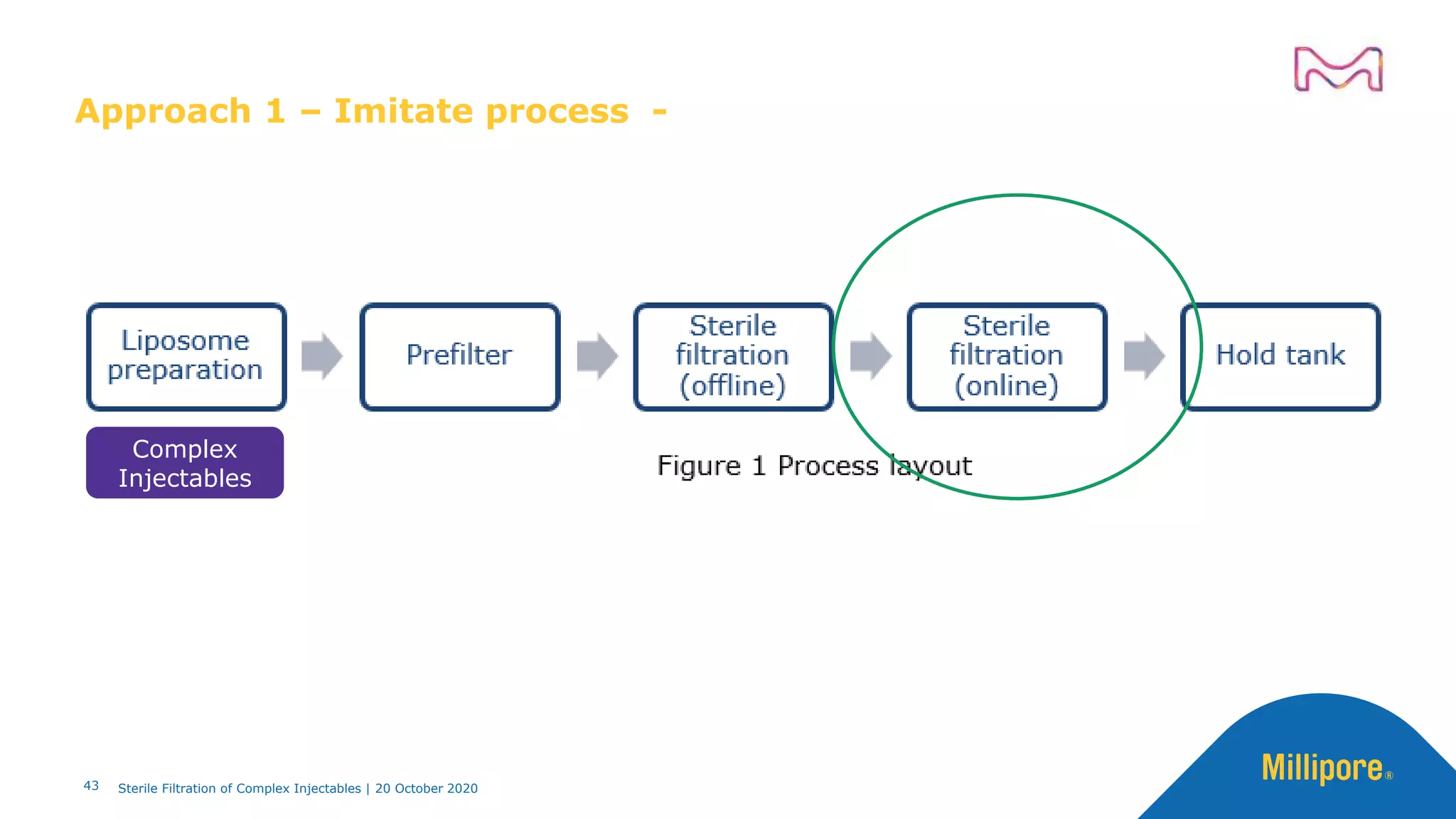
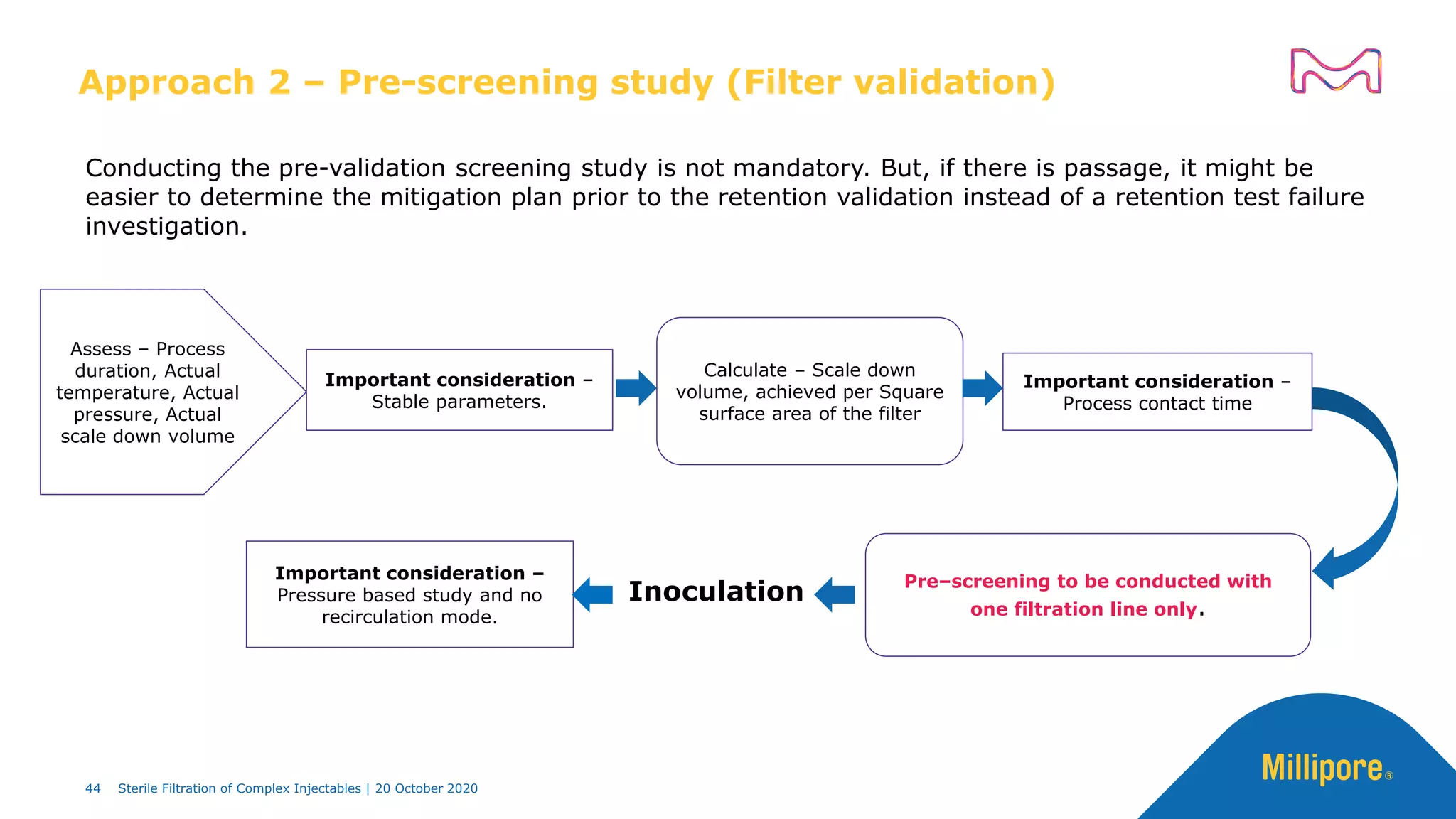
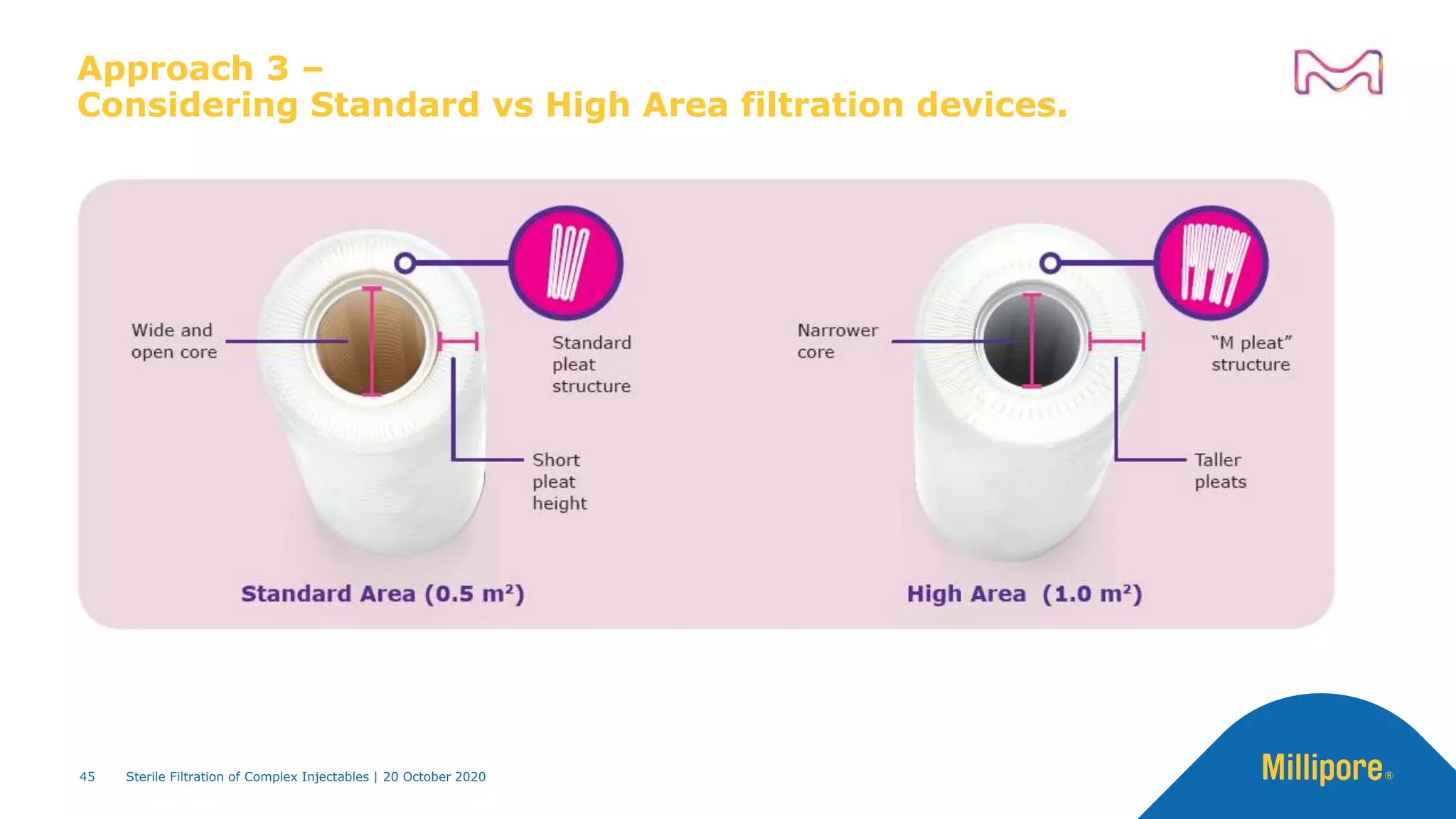
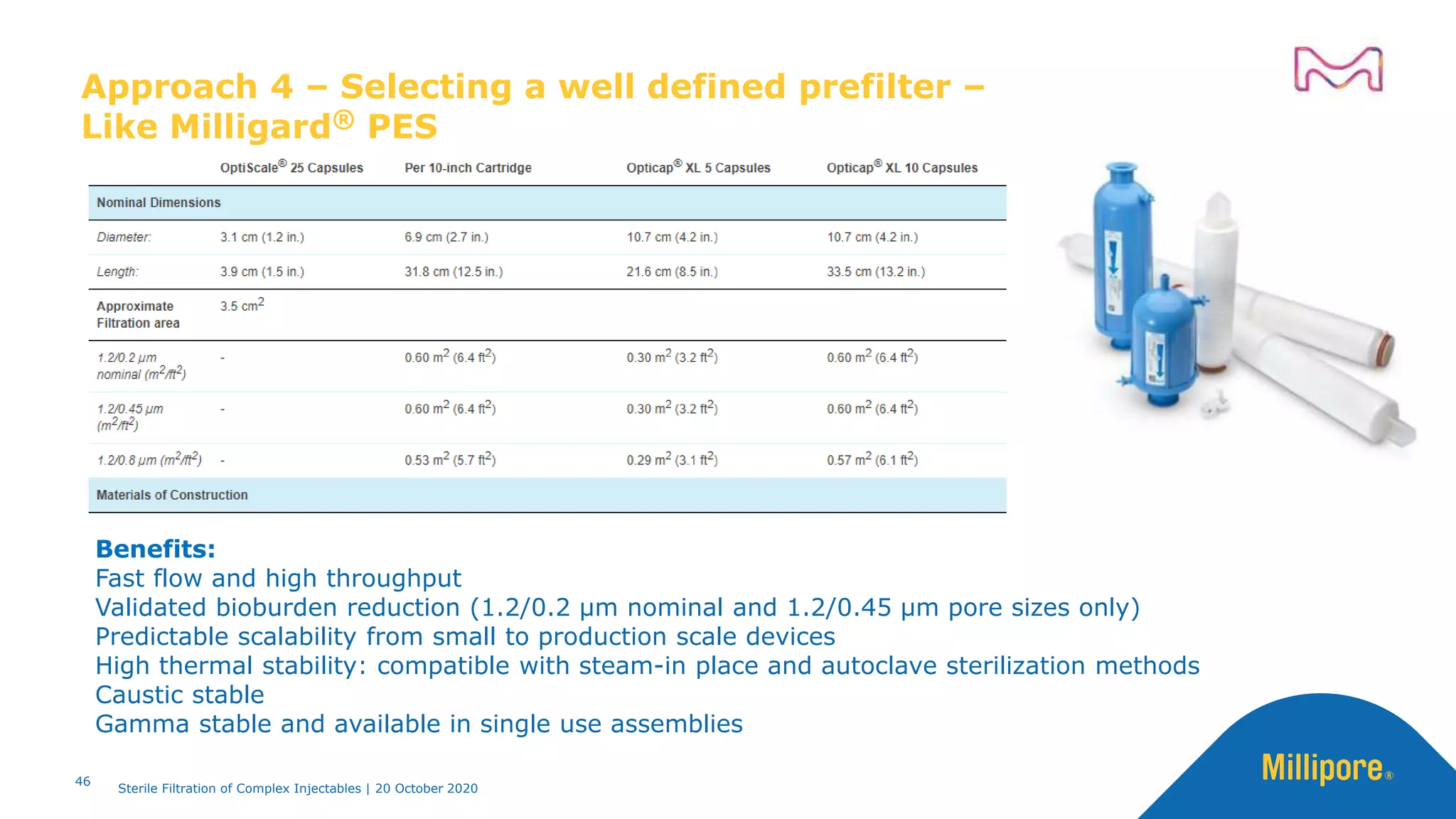
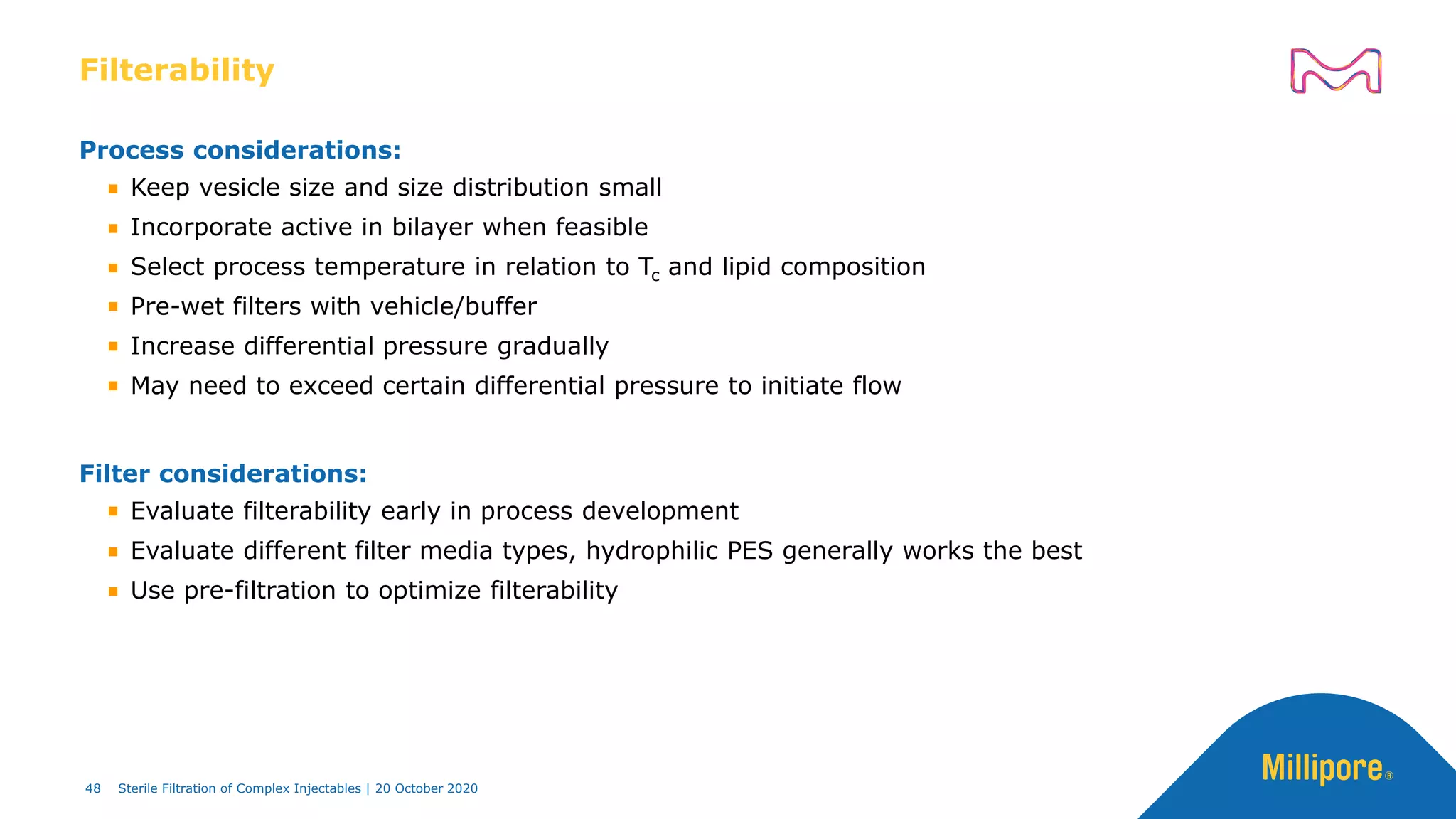
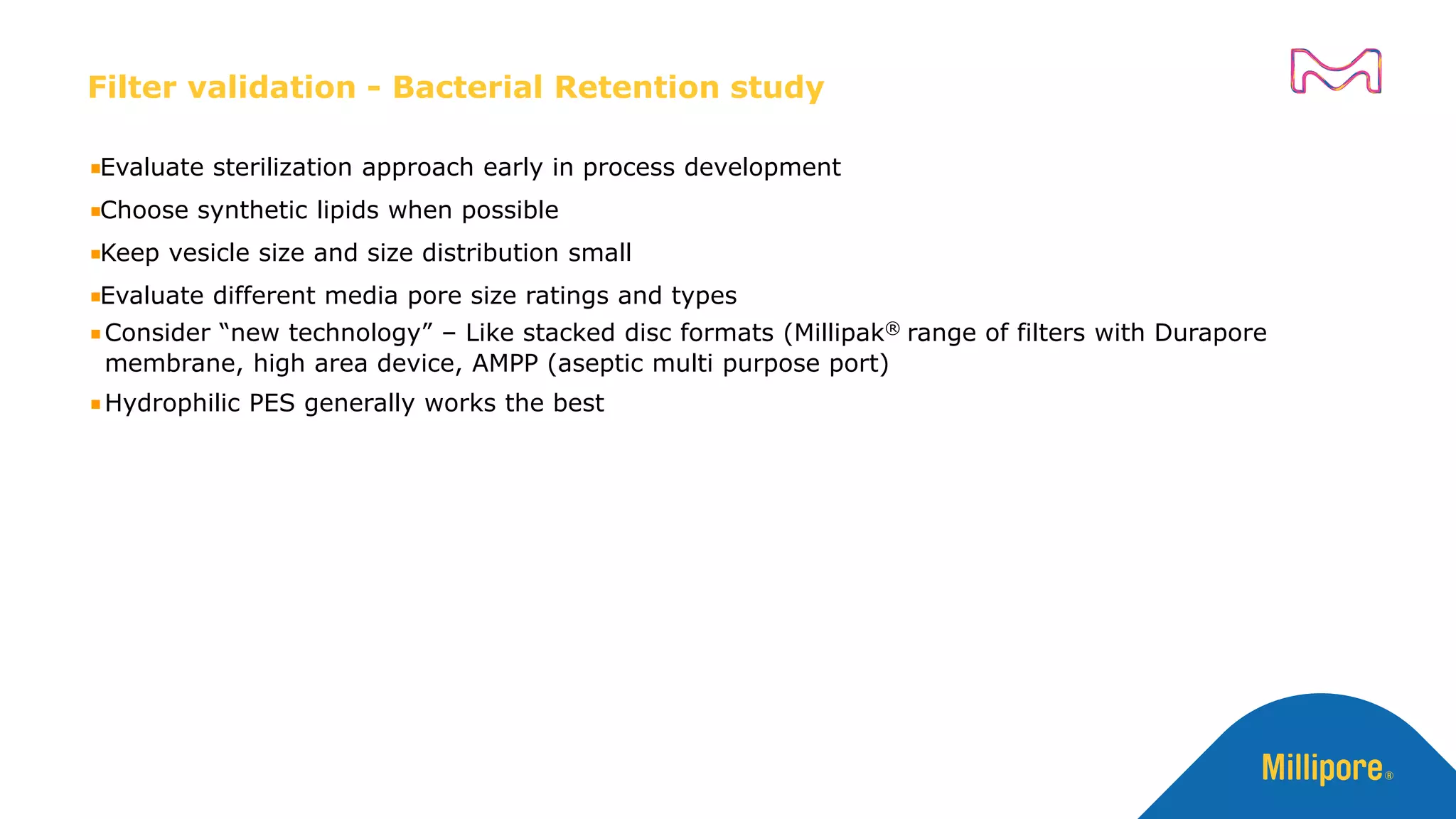
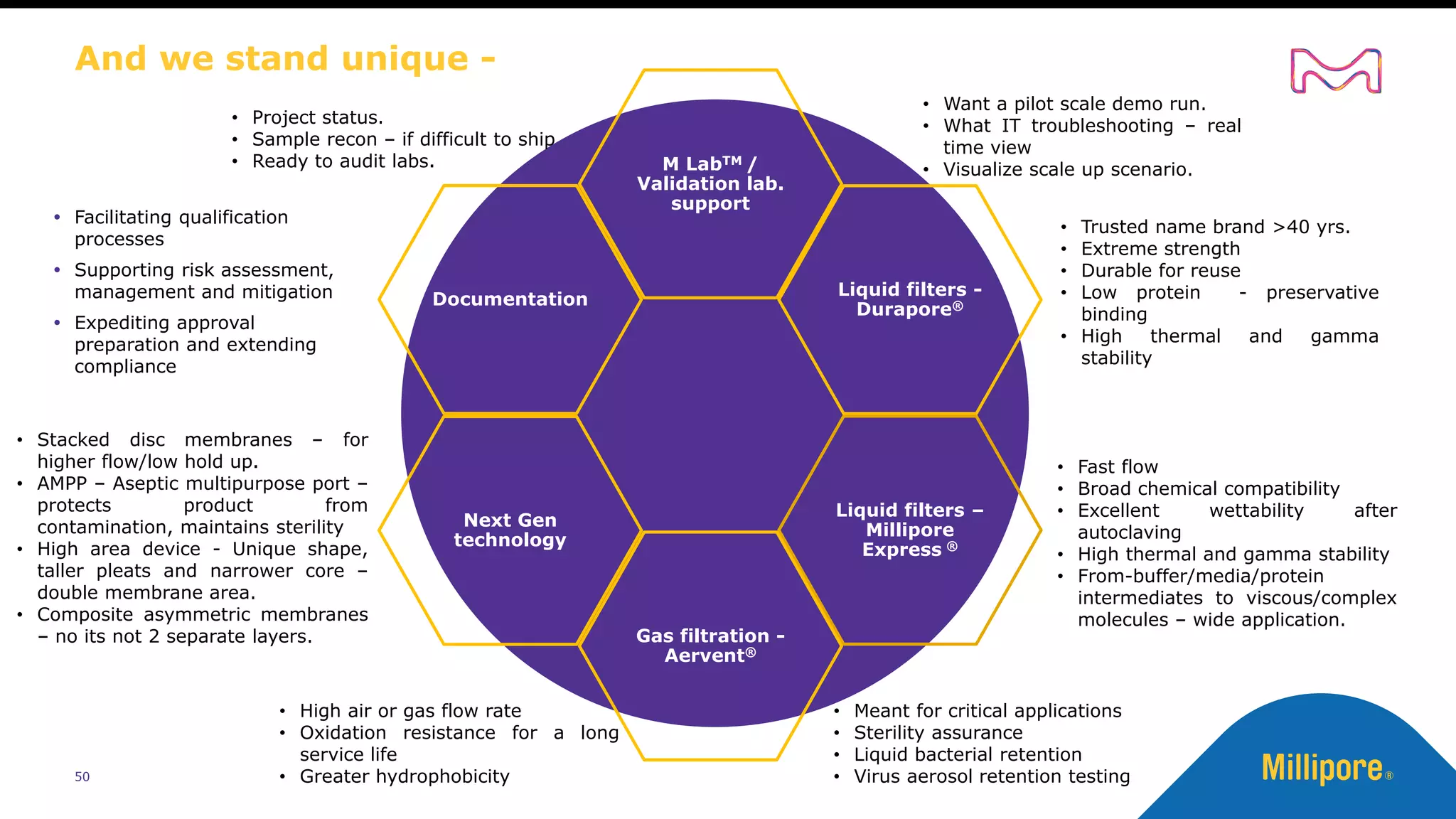
The document discusses considerations for sterile filtration of complex injectables such as liposomes, emulsions, and viscous formulations. It notes that sterile filtration is widely used for liposome sterilization but poses challenges due to the small size of liposomes and bacteria. Key parameters for liposomes include particle size distribution, zeta potential, and stability. The talk addresses regulatory guidelines for liposome characterization and manufacturing. It also outlines best practices for sterile filtration of oils, emulsions and viscous drugs to optimize the filtration process.



![Complex injectables – what are they?
Complex drug products have become so prevalent that the FDA has defined them with the following
categories:
• Products with complex active ingredients (e.g., peptides, polymeric compounds, complex mixtures of
[active pharmaceutical ingredients]); complex formulations (e.g., liposomes, colloids); complex routes
of delivery (e.g., locally acting drugs, complex ophthalmological products and otic dosage forms that
are formulated as suspensions, emulsions, or gels); or complex dosage forms (e.g., implantables,
transdermals, metered dose inhalers, extended-release injectables
• Complex drug-device combination products (e.g., auto-injectors)
• Other products where complexity or uncertainty concerning the approval pathway or possible
alternative approach would benefit from early scientific engagement.
Complex processing challenges include, among others, aseptic manufacturing, the inclusion of highly
potent compounds, milling/particle engineering, spray drying, extrusion, and microfluidization.
6
Ref: Synthetic peptide drug products that refer to listed drugs of rDNA origin, Draft guidance, October 2017.
Sterile Filtration of Complex Injectables | 20 October 2020](https://image.slidesharecdn.com/sterilefiltrationofcomplexinjectablesbyparthabanerjee-201021071833/75/Sterile-filtration-of-complex-injectables-by-Partha-Banerjee-6-2048.jpg)