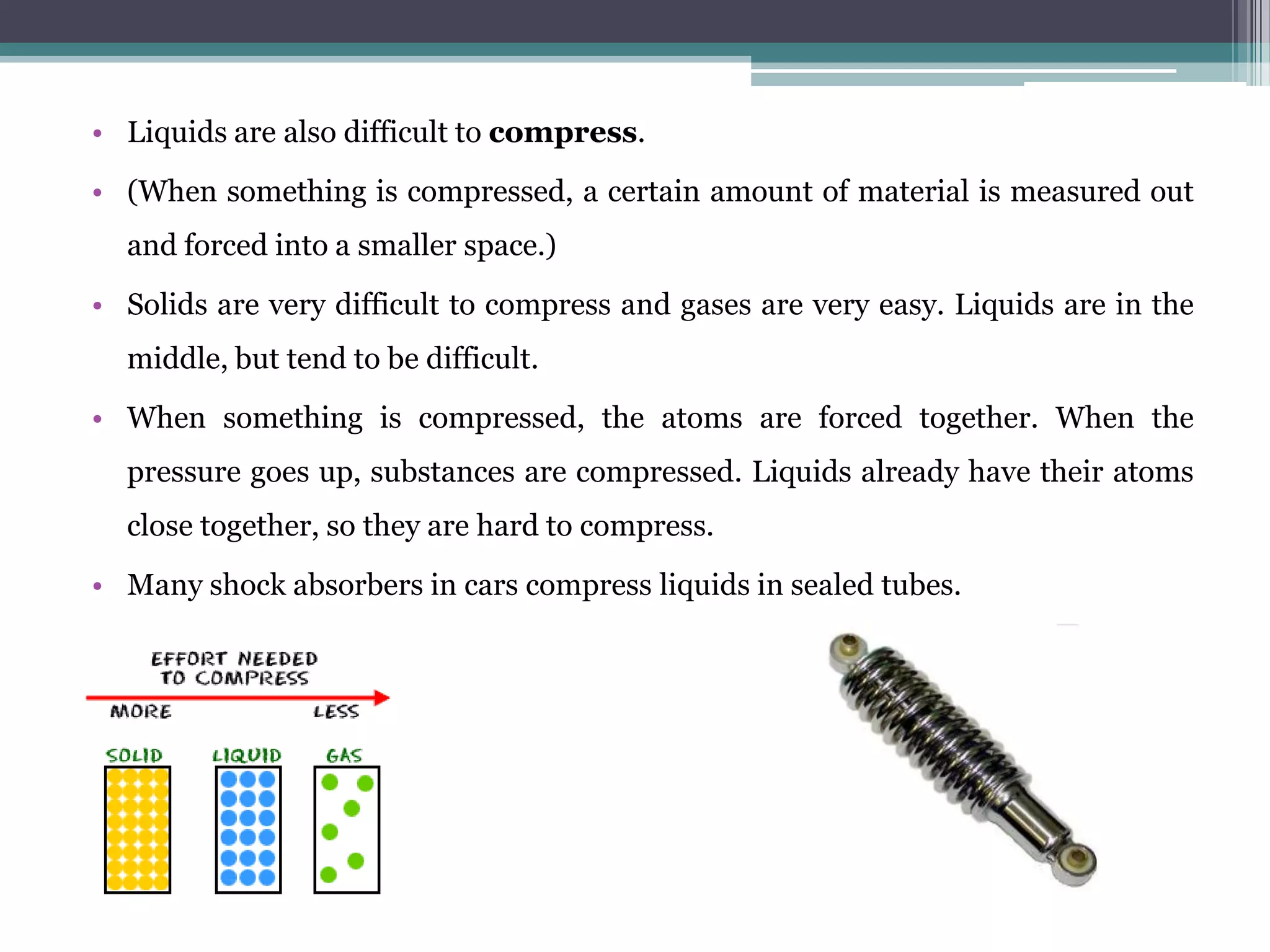
Liquids have specific properties that distinguish them from solids and gases. They take the shape of their container but maintain a relatively constant volume. Key liquid properties include viscosity, surface tension, vapor pressure, boiling point, freezing point, capillary action, miscibility, and osmosis. Viscosity is a liquid's resistance to flow, while surface tension allows some objects to float on water. Vapor pressure and boiling point are related, as boiling occurs when vapor pressure equals atmospheric pressure. Capillary action and osmosis involve the passage of liquids through membranes.















![References
• http://hyperphysics.phy-astr.gsu.edu/hbase/kinetic/vappre.html
• http://library.thinkquest.org/20331/physics/vapor.html
• http://www.chemprofessor.com/liquids.htm
• http://www.edinformatics.com/math_science/states_of_matter.htm
• http://en.wikipedia.org/wiki/Viscosity
• http://www.chem4kids.com/files/matter_liquid.html
• http://www.chem.purdue.edu/gchelp/atoms/states.html
• ―Liquid.‖ Microsoft ® Encarta ® 2009 [DVD]. Redmond, WA: Microsoft
Corporation, 2008.](https://image.slidesharecdn.com/statesofliquidmatter-121017120514-phpapp01/75/States-of-Liquid-Matter-17-2048.jpg)