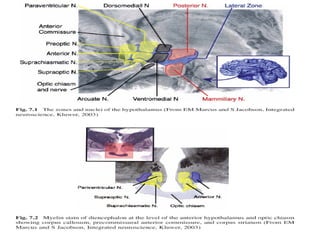
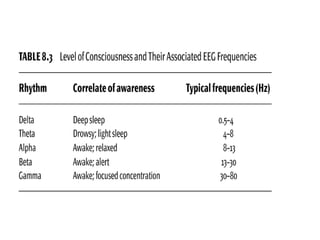
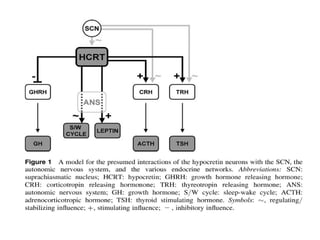
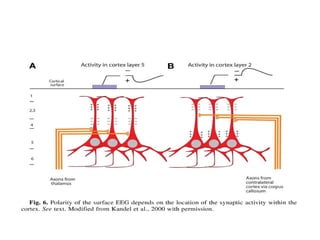
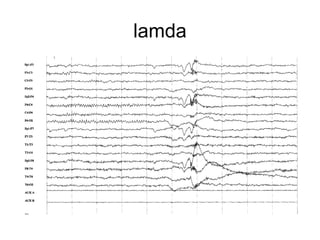
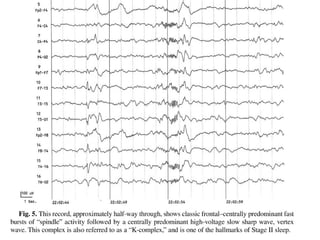
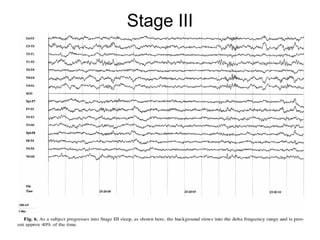
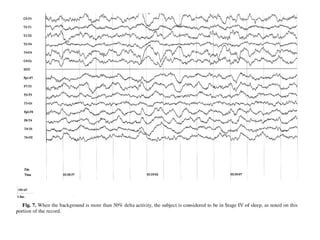
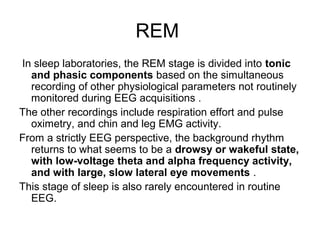
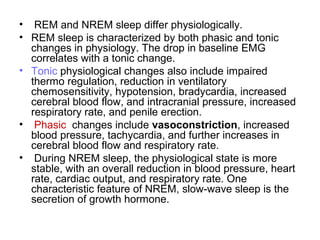
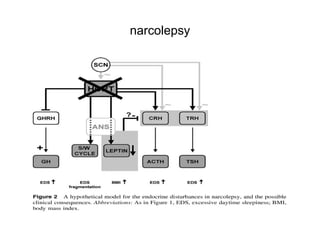
The document discusses biological rhythms and sleep-wake cycles. It describes circadian rhythms controlled by the suprachiasmatic nucleus and influenced by factors like light and temperature. Two drives regulate sleep - a homeostatic drive that increases with wakefulness, and a circadian drive controlled by the suprachiasmatic nucleus. Neurons in the ventrolateral preoptic nucleus promote sleep by inhibiting wake-promoting areas. The document also discusses EEG patterns in different sleep stages, including spindles in stage 2 and delta waves in stage 3. It mentions the role of orexin/hypocretin neurons in maintaining wakefulness and inhibiting REM sleep.