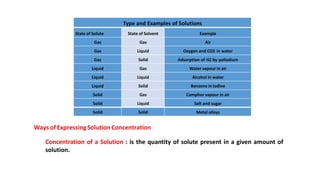
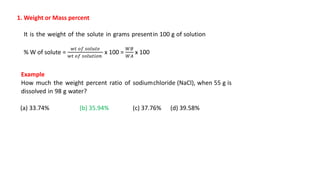
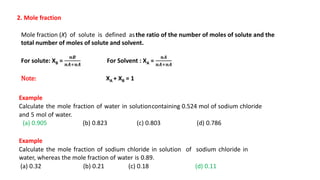
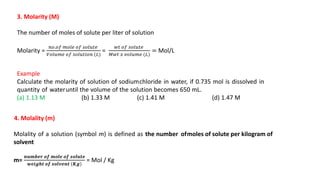
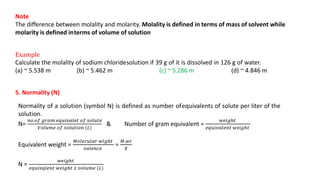
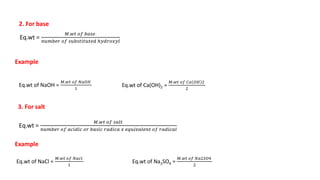
There are several types of solutions depending on the state of the solute and solvent. The main types are gas-gas, gas-liquid, gas-solid, liquid-gas, liquid-liquid, liquid-solid, solid-gas, solid-liquid, and solid-solid solutions. Concentration of a solution can be expressed in various ways including weight/mass percent, mole fraction, molarity, molality, normality, strength, parts per billion (ppb), and parts per million (ppm). These units of concentration allow the amount of solute present in a solution to be quantified.