Embed presentation

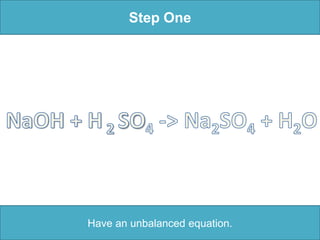
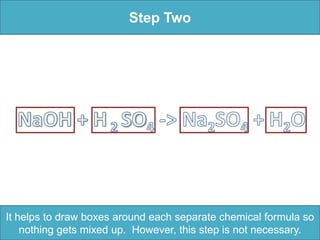
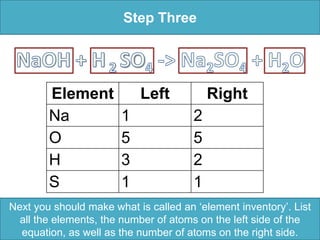

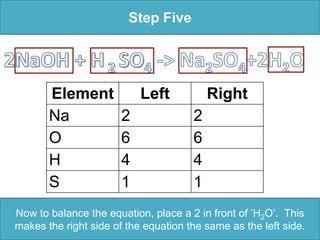


To balance a chemical equation: 1. Make an element inventory of the atoms on each side of the equation. 2. Adjust coefficients as needed to make the number of each element equal on both sides. 3. For the example equation, placing coefficients of 2 in front of NaOH and H2O balanced the numbers of each element on both sides.






