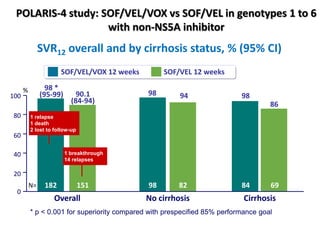
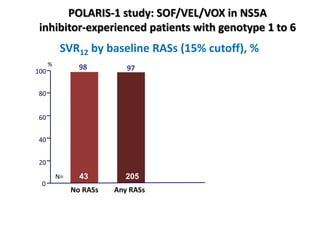
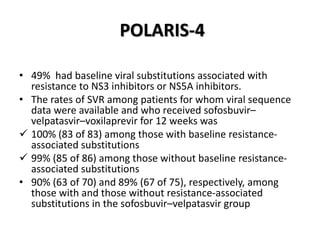
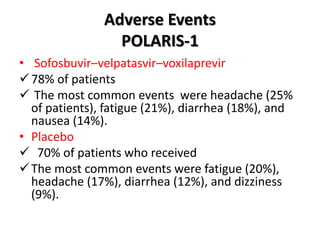
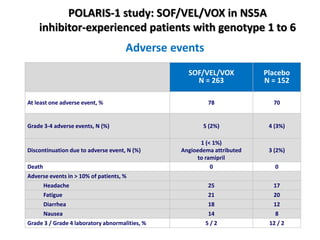
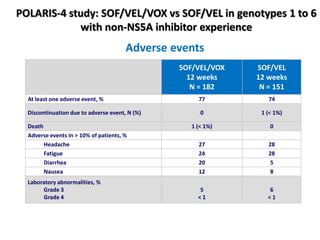
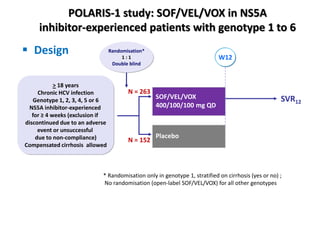
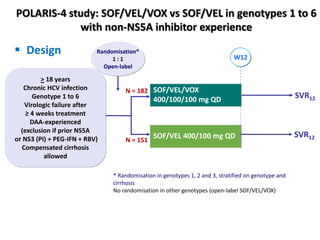
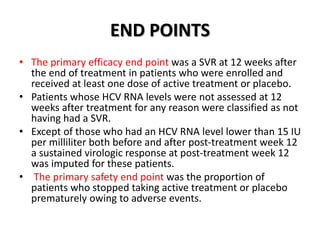
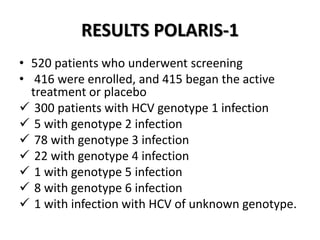
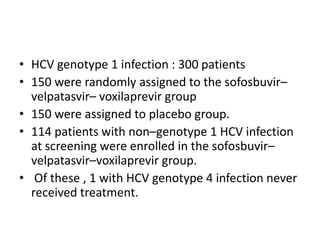
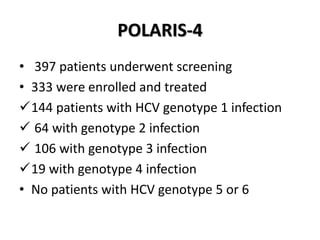
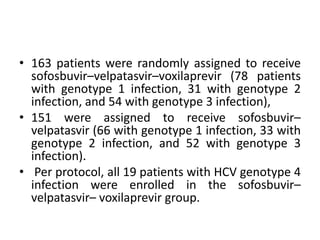
The document summarizes results from two phase 3 clinical trials that assessed the efficacy and safety of a fixed-dose combination of sofosbuvir, velpatasvir, and voxilaprevir for 12 weeks in patients with hepatitis C virus (HCV) who had previously received unsuccessful treatment with direct-acting antiviral (DAA)-based regimens. In POLARIS-1, the overall sustained virologic response rate was 96% among those receiving sofosbuvir, velpatasvir, and voxilaprevir, significantly higher than the prespecified goal of 85%. In POLARIS-4, patients receiving sofosbuvir, velpatasvir, and voxilap














![EFFICACY POLARIS-1
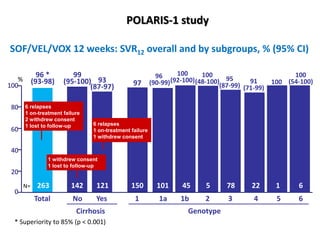
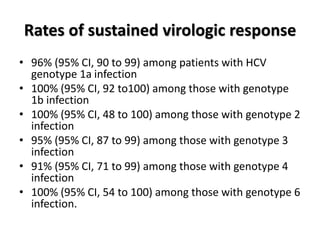
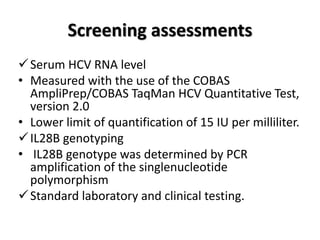
• The overall rate of SVR in the sofosbuvir–velpatasvir–
voxilaprevir group was 96% (95% confidence interval
[CI], 93 to 98), which was significantly superior to the
pre specified performance goal of 85% (P<0.001)
• Of the 253 patients with SVR 12 week after treatment,
i. 249 returned for the post-treatment week 24 visit.
ii. All 249 patients had a SVR at that time.
• None of the patients who received placebo had a SVR.](https://image.slidesharecdn.com/sofosbuvirppt-170906160043/85/Sofosbuvir-ppt-30-320.jpg)