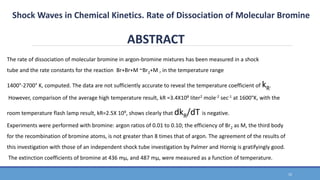
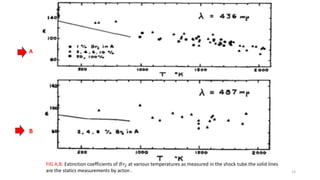
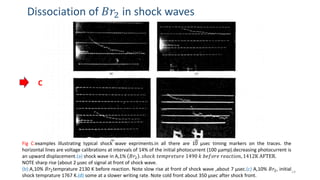
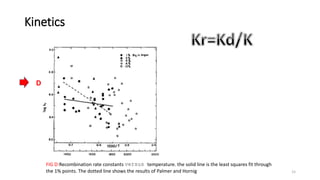
The document discusses shock waves and shock tubes, providing definitions and applications in various fields such as medicine and chemical kinetics. It presents experimental results on the rate of dissociation of molecular bromine under shock conditions, detailing the methodologies used and comparing results with existing literature. The findings emphasize the interaction of shock waves with different gas mixtures and their significance in simulating explosive reactions.