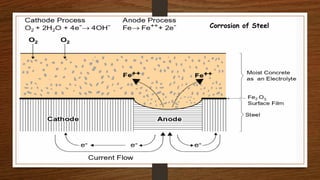
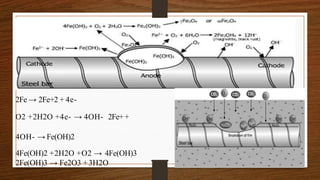
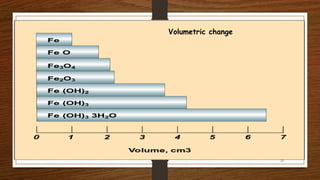
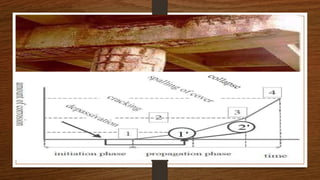
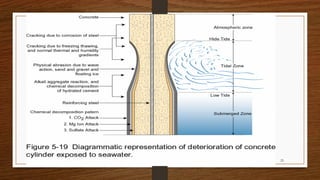
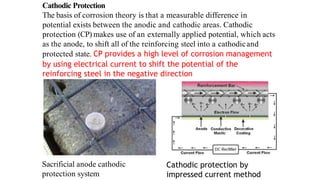
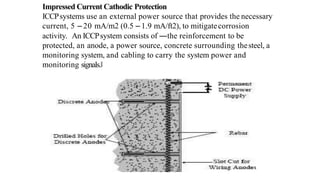
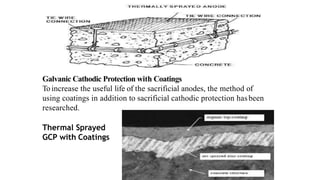
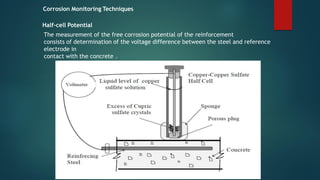
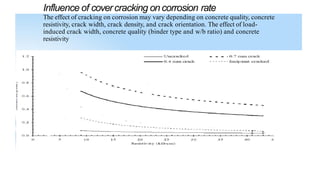
This document discusses factors that influence the serviceability and durability of concrete, including climate, temperature, chemicals, wear and erosion, design errors, and corrosion. It covers the effects of cover thickness and cracking, as well as methods of corrosion protection like corrosion inhibitors, corrosion resistant steels, coatings, and cathodic protection. The document also examines causes of corrosion like carbonation, chloride penetration, and sulfate attack, and methods to improve concrete durability.