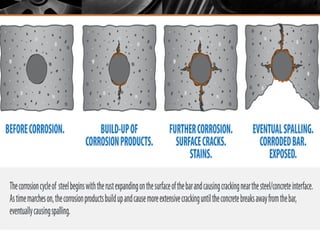
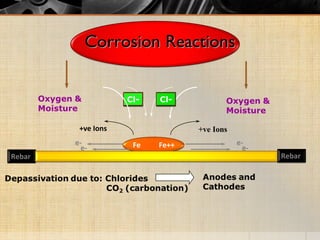
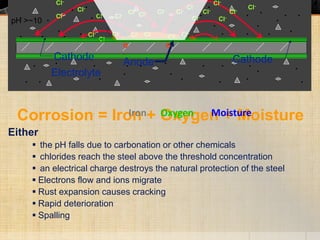
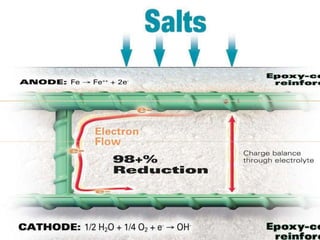
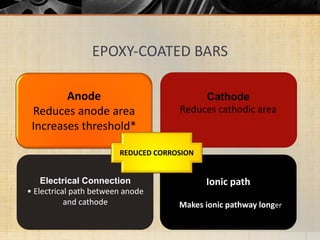
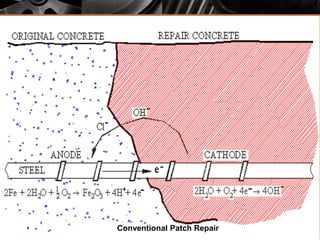
The document summarizes corrosion of steel reinforcement in concrete. It defines corrosion and describes the types as crevice and pitting corrosion. Chlorides are identified as the main cause as they can penetrate the protective oxide layer on the steel. Carbonation is also discussed as it lowers the pH and exposes the steel. The consequences of corrosion are outlined as rust formation which causes cracking, spalling and structural damage. Methods to prevent corrosion include coatings on the steel, using fly ash, galvanizing, and monitoring chlorides. Repair methods involve removing loose concrete, cleaning steel, applying protective coatings, and cement or epoxy patching.