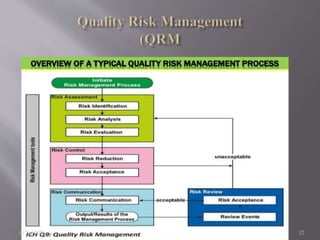
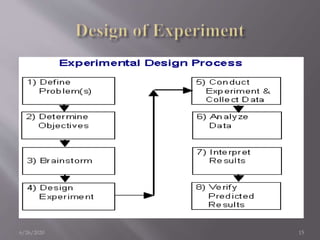
This document summarizes key concepts in quality by design (QbD) for pharmaceutical product and process development. It discusses that QbD is a systematic approach that begins with predefined quality targets and uses product and process understanding to establish design space and process controls. The document outlines critical elements of QbD including quality target product profile, critical quality attributes, critical material attributes, critical process parameters, design space, risk assessment, and design of experiments. It provides examples of each element and explains how they guide development and ensure quality.