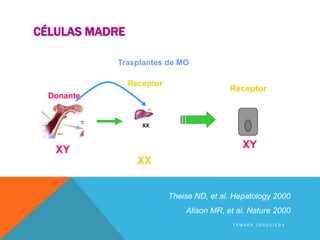
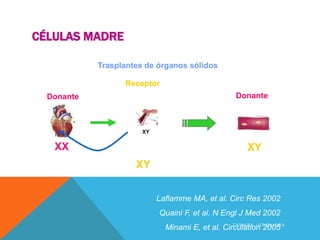
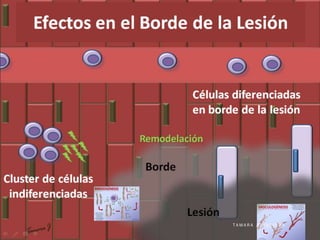
This study investigated whether mesenchymal stem cells (MSCs) could regenerate chronically infarcted myocardium through long-term engraftment and trilineage differentiation. MSCs were injected into infarcted pig hearts and were found to engraft in the infarct and border zones. The MSCs differentiated into cardiomyocytes, vascular smooth muscle cells, and endothelial cells, as evidenced by co-localization with lineage markers. MSC treatment reduced infarct size and increased ejection fraction and blood flow compared to placebo. Engraftment of MSCs correlated with improvements in contractility and blood flow. This demonstrates that MSCs can survive long-term after transplantation, engraft in scarred heart tissue, and