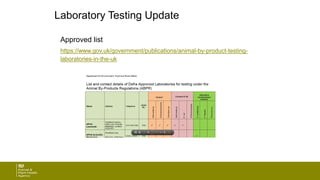
The document provides an update on regulations regarding animal by-products from the Animal and Plant Health Agency (APHA). It discusses changes to laboratory testing requirements, definitions of digestate, and potential future charging for approvals and inspections. Guidance documents have also moved to the Gov.UK website and have been revised in format, language, and detail.