The document provides an overview of topics covered in Grade 9 Science Term II, including atoms and molecules, atomic structure, diversity of living organisms, work and energy, and sound. Key points include: the basic structure of atoms; atomic models like Rutherford and Bohr models; principles of classification of living organisms; the five kingdoms of classification; and definitions of work, energy, power, and the characteristics of sound waves like frequency, wavelength, amplitude. Plant and animal kingdoms are described in detail including major phyla in each kingdom.




![o Warm-blooded animals with four chambered heart
o They breathe through lungs
o Have feathers and forelimbs modified for flight
o Exclusively egg-laying animals
Class Mammalia: Includes kangaroo, rat, dolphin, elephant, horse, human, tiger, etc
o Warm-blooded animals with four chambered heart
o Most of them are viviparous except for platypus and echidna. They both are
oviparous
o These animals have milk-producing glands (mammary glands) to nourish their young
ones
Chapter 4: Work and Energy
Scientifically, work is done when:
There is a displacement.
Displacement is in any direction except the direction normal to the direction of force.
No work is done when
Net displacement is zero. [No work is done in circular path]
Displacement occurs perpendicularly to the applied force
Work = Force Displacement [along force direction]
W = F s [Unit – Joule, 1 J = 1 N.m]
Unit of energy: Joule
Commercial unit of Energy: kWh
1 kWh 3.6 106 J
The energy possessed by a body by virtue of its motion is called kinetic energy.
1
Kinetic energy of a body = mv 2 , where m is mass and v is speed of the body.
2
Proof:
v 2 u 2 2as
v2 u 2
s
2a
v2 u 2
W ma
2a
1
2
m v 2 u 2 mv 2 when u 0
1
2
Energy possessed by a body by virtue of its position or its shape is called potential energy.
Gravitational potential energy = mgh where, m is mass, g acceleration due to gravity, and h is
the height above surface of Earth.
Law of conservation of energy: Energy can neither be created nor destroyed, it is only
converted from one form to other.](https://image.slidesharecdn.com/sciencesa2synopsis-121001041010-phpapp01/85/Science-sa-2-synopsis-6-320.jpg)
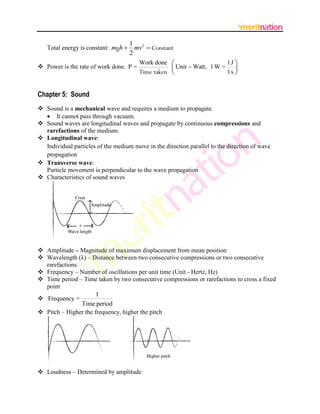
![ Tone – Sound of single frequency
Speed of sound depends on temperature, pressure, humidity and nature of the material
medium.
Speed increases with increasing temp.
Speed in solid > Speed in liquid > Speed in gas
In air, speed 344 m s–1 at 22 C
Supersonic – More speed than sound
Sonic boom loud noise produced by supersonic object is sonic boom
Echo- Reflection of sound
1
Sensation of sound persists = 0.1 s in the human brain
10
344×0.1
Minimum distance to hear echo = = 17.2 m
2
Reverberation – Persistence of sound by repeated reflection
Uses – Loud speaker, stethoscope, curved ceiling of a concert hall, sound board in a big
hall
Range of hearing for humans: 20 – 20000 Hz
But, rhinoceroses use infrasound
Application of ultrasound : Cleaning, detecting defects in metals, echocardiography,
ultrasonography, to break small kidney stone
SONAR is Sound navigation and Ranging.
Human ear: Pinna collects sound; eardrum vibrates in response to sound
Vibrations are amplified by the three ear bones [hammer, anvil, stirrup (smallest human
bone)]
Chapter 6: Why Do We Fall Ill
Health: A state of physical, mental, and social well-being, which includes a unity and
harmony within the mind, body, and soul of an organism
Disease: Any condition that can lead to discomfort, distress, health problems, and even death
of the affected person
Symptoms: Indications of disease, such as headache, stomach pain, nausea, etc that can only
be felt by the patient
Signs of a disease include fever, vomiting, diarrhoea, etc that can be observed by a physician
Incubation period: The time interval between infection and appearance of symptoms
Causes of diseases](https://image.slidesharecdn.com/sciencesa2synopsis-121001041010-phpapp01/85/Science-sa-2-synopsis-8-320.jpg)