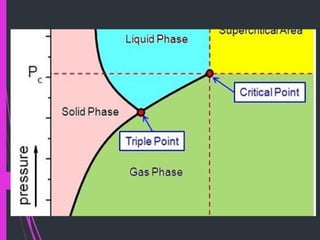
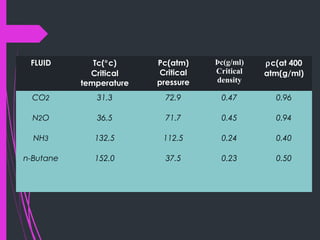
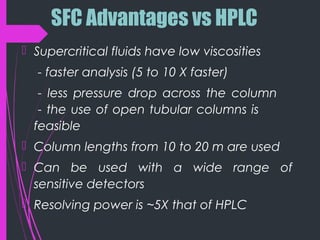
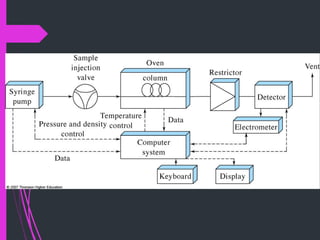
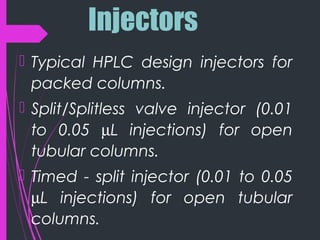
Super critical fluid chromatography (SFC) is a form of normal phase chromatography that uses carbon dioxide as the mobile supercritical fluid. It can be used for the analysis and purification of low to moderate molecular weight and thermally labile molecules. SFC provides faster analysis times than HPLC and can analyze a wider range of compounds than GC without derivatization. Detectors commonly used include UV and mass spectrometry. Columns can be open tubular or packed, with carbon dioxide being the most common solvent due to its low critical temperature, moderate critical pressure, and compatibility with detectors. Organic modifiers are added to carbon dioxide to modify solvent strength for different compounds.