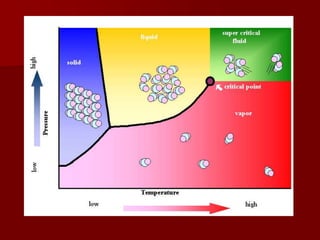
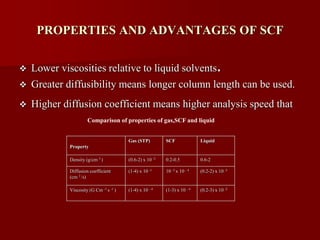
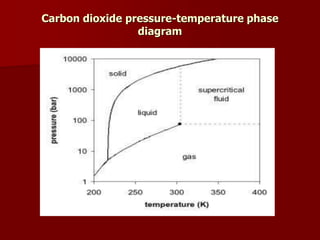
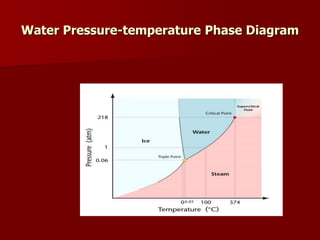
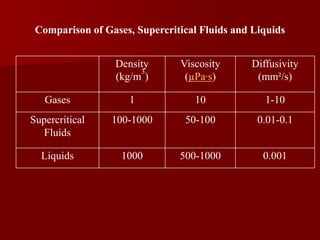
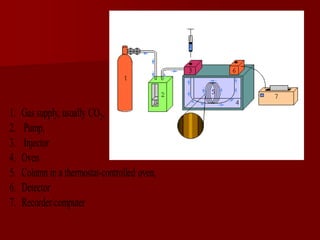
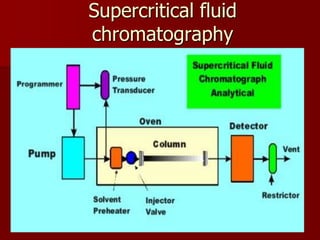
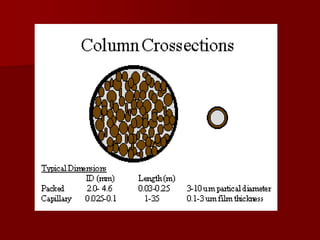
Supercritical Fluid Chromatography (SFC) is a technique that uses supercritical fluids as the mobile phase to efficiently separate chemical mixtures. Supercritical fluids, like carbon dioxide and water, possess unique properties that enhance solubility and analysis speed, making SFC superior to traditional chromatographic methods. SFC is used in various applications, including the analysis of natural products, drugs, foods, and chiral compounds.