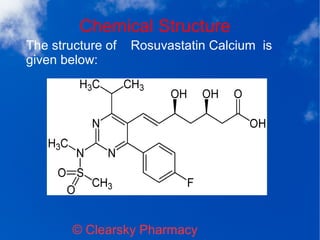
Rosuvastatin calcium tablets, marketed as rostar, are used to treat high cholesterol and lower the risk of heart attack and stroke in patients with multiple risk factors. The typical dosage ranges from 5 to 40 mg daily, and the medication should be monitored for potential side effects like muscle pain or liver enzyme abnormalities. It is contraindicated for pregnant or nursing women and those with known hypersensitivity to its components.