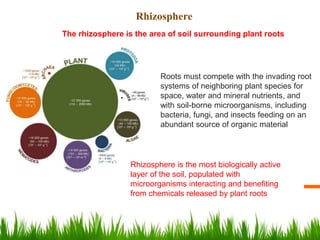
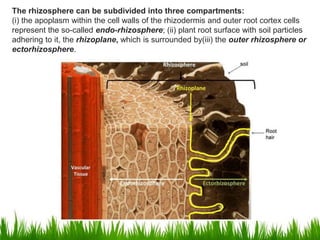
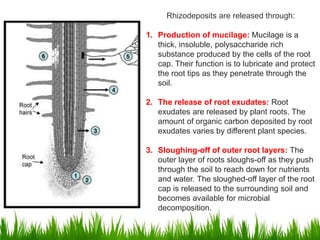
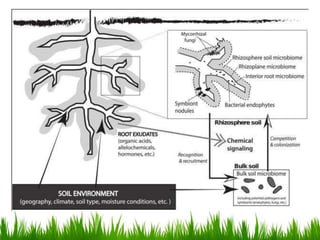
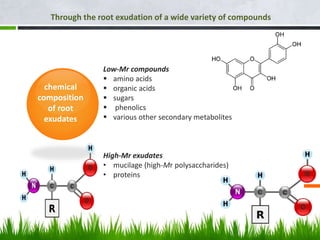
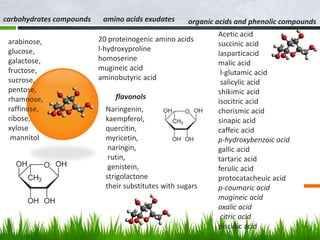
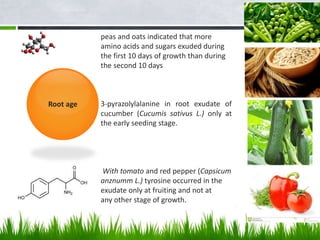
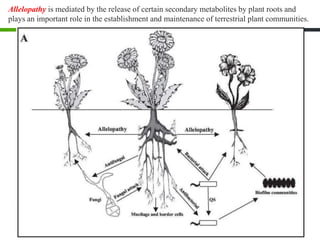
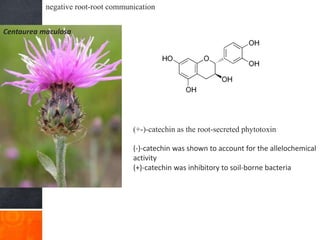
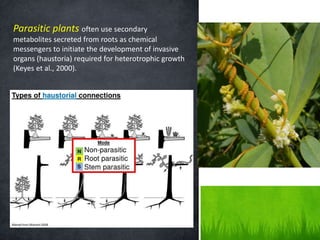
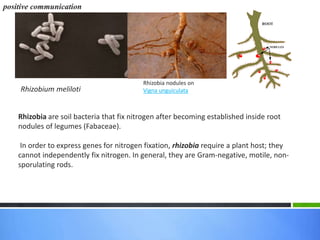
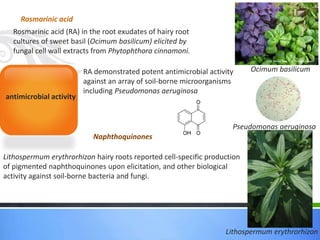
Roots release chemicals called rhizodeposits into the surrounding soil, known as the rhizosphere. This biologically active area is populated by microorganisms that interact with and benefit from root exudates. Rhizodeposits include mucilage, root exudates, and sloughed-off root cells and layers. They support soil microbial life and their composition varies by plant type, climate, soil properties, and nutrient availability. Root exudates communicate with both microbes and other plant roots, selectively fostering beneficial microbes while inhibiting competing plants or pathogens through allelopathy. The rhizosphere is crucial for nutrient acquisition and microbial symbioses that are important for plant growth and health.