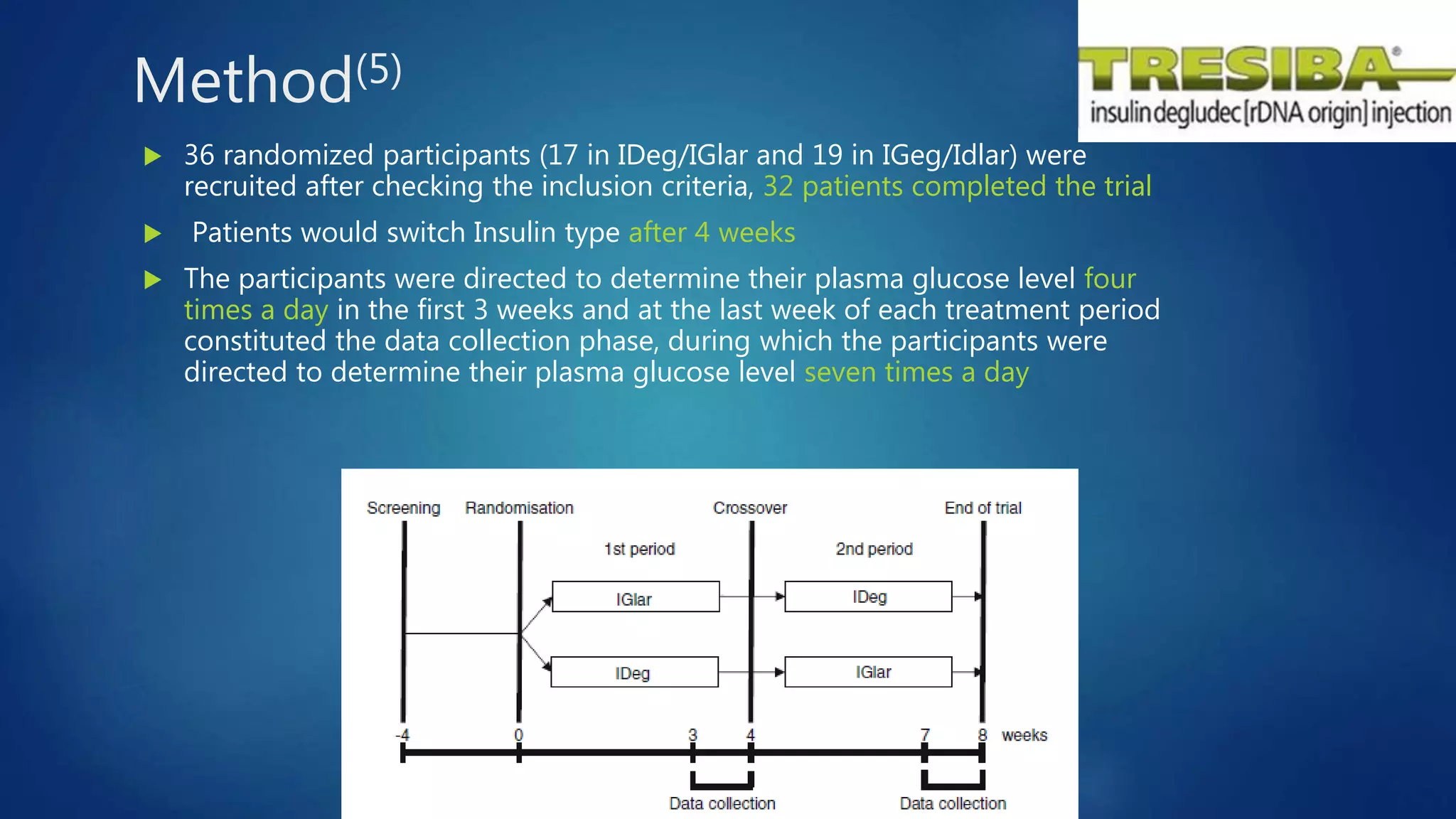
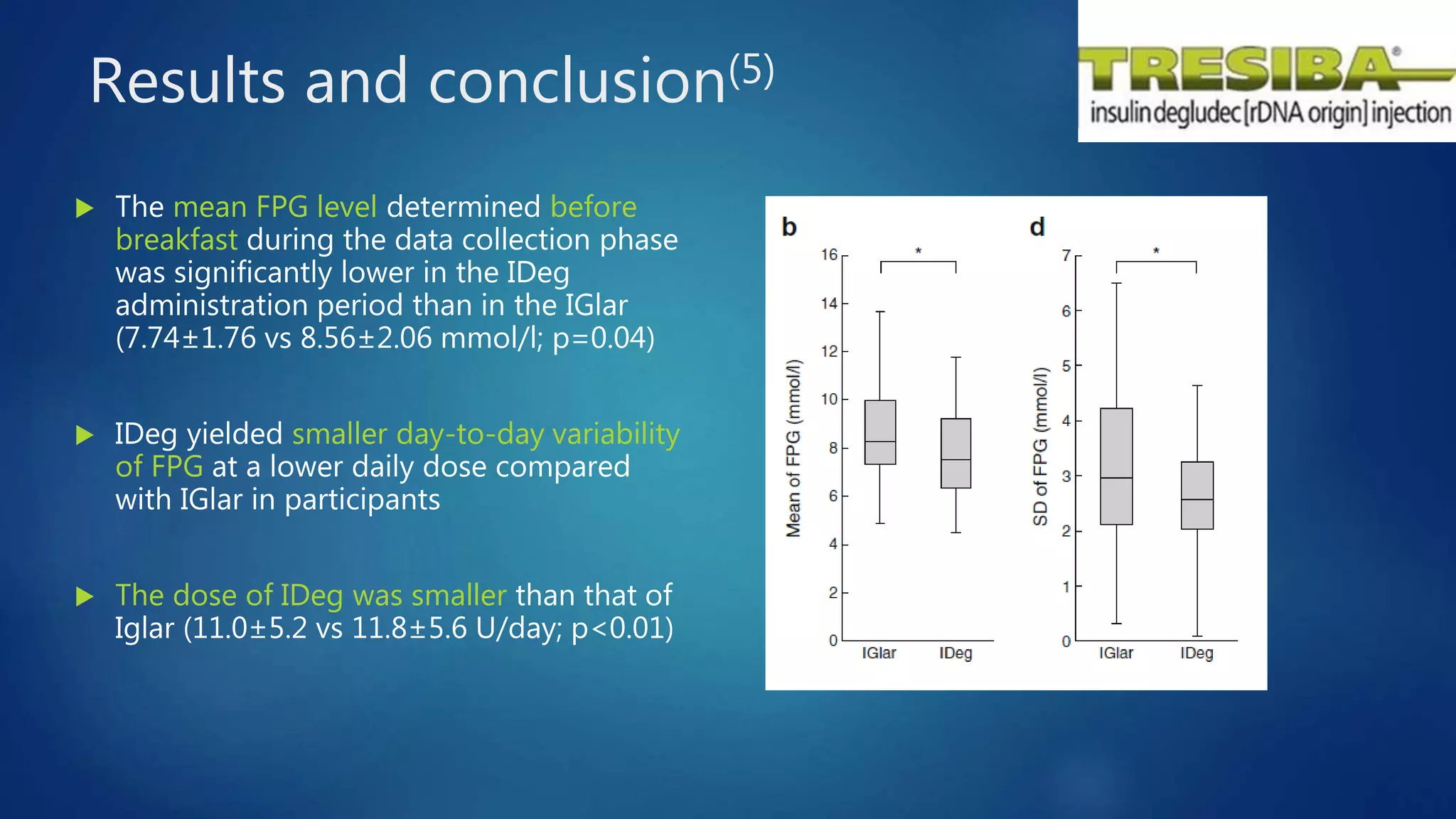
The document reviews studies of new insulin products including Degludec (Tresiba), Degludec/Aspart (Ryzodeg), and Glargine (Basaglar). It finds that Degludec has a longer duration of action of over 42 hours and lower day-to-day variability compared to other long-acting insulins. Degludec/Aspart is found to reduce post-dinner blood glucose excursions and provide more stable nocturnal glycemia than Glargine. Basaglar is approved as the first follow-on biologic insulin and demonstrated comparable efficacy and safety to Glargine in clinical trials.













![Results and conclusion (6)
The postdinner IG increment observed with IGlar did not occur
with IDegAsp [IDegAsp - IGlar, -1.42 (-2.15, -0.70) mmol/liter].
Nocturnal IG fluctuation was 21% lower with IDegAsp [IDegAsp/IGlar, 0.79 (0.66,
0.96) mmol/liter], with 48% fewer nocturnal high IG episodes [ratio IDegAsp/IGlar,
0.52 (0.32, 0.87)].
IDegAsp given with the evening meal reduces postdinner glucose excursion and
provides more stable nocturnal glycemia as compared with IGlar.](https://image.slidesharecdn.com/reviewstudiesofnewinsulinproducts-170313161444/75/Review-studies-of-new-insulin-products-16-2048.jpg)





