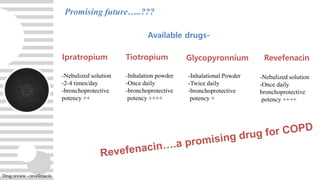
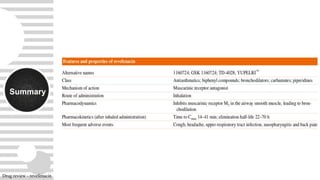
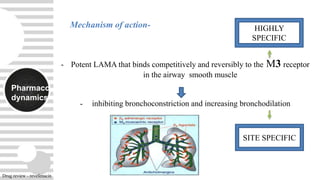
Revefenacin is a once-daily nebulized long-acting muscarinic antagonist approved for the maintenance treatment of COPD that works by selectively inhibiting bronchonstriction-causing M3 receptors in the lungs. It was found to be generally well-tolerated in clinical trials with common side effects including cough and headache. Revefenacin addresses a need for additional nebulized maintenance treatment options for COPD patients who have difficulty using handheld inhaler devices.

![REVEFENACIN
[YUPELRI]](https://image.slidesharecdn.com/revefenacindrugreview-190729174154/85/Revefenacin-2-320.jpg)
![Introduction
- The mainstay maintenance treatment for COPD patients includes inhaled long-acting
muscarinic antagonist [LAMAs] and/or long-acting β2-agonists (LABAs) with or without
Inhaled corticosteroid
- Long-acting bronchodilators (e.g. LABAs or LAMAs) are often delivered via hand-held
devices, such as a dry powder inhaler (DPI) or a metered dose inhaler (MDI).
- However, some COPD patients [e.g. the elderly, cognitively impaired] find it difficult to use
such devices, resulting in inaccurate dosing, poor adherence, and potentially poor clinical
outcomes
- For these patients, nebulized drug delivery, which is easier to use and provides similar
efficacy to hand-held inhalers can be an alternative treatment option.
- Nebulized bronchodilator therapy options for the maintenance treatment of COPD are
currently limited
Current treatment of COPD-
Drug review - revefenacin](https://image.slidesharecdn.com/revefenacindrugreview-190729174154/85/Revefenacin-4-320.jpg)



![Pharmaco
kinetics
-
- Following administration of a single radiolabeled oral dose of revefenacin, 88% of total
radioactivity was recovered in the feces and < 5% was present in urine, suggesting low oral
absorption. There was minimal renal excretion after inhaled revefenacin [<5%]
Dosage adjustments-
- systemic exposure are not significantly affected by age, gender,
smoking status or weight
- Revefenacin is not recommended in patients with any degree of hepatic impairment as the safety
of revefenacin has not been studied in COPD patients with mild to severe hepatic impairment
- Dosage adjustments are not required in patients with renal impairment
Excretion of the drug-
Drug review - revefenacin](https://image.slidesharecdn.com/revefenacindrugreview-190729174154/85/Revefenacin-9-320.jpg)
![Adverse
events
- Revefenacin inhalation solution was generally well tolerated in patients with moderate to very
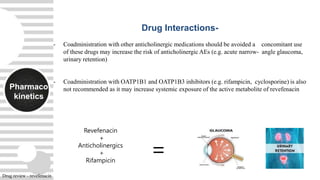
severe COPD in clinical trials [ caution in narrow angle glaucoma patients]
- The most common AEs occurring in ≥ 2% of patients with a higher incidence in revefenacin
88 or 175 μg in recipients were-
- cough 4.0%
- headache 2.6%
- upper respiratory tract infection 2.1%
- nasopharyngitis 2.1%
- back pain 1%
- The incidence of antimuscarinic adverse events were low
Drug review - revefenacin](https://image.slidesharecdn.com/revefenacindrugreview-190729174154/85/Revefenacin-11-320.jpg)