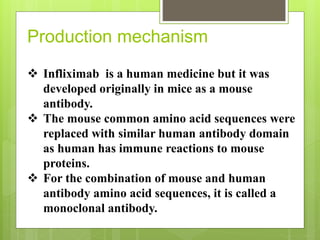
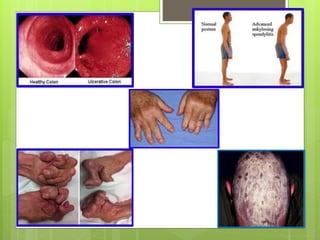
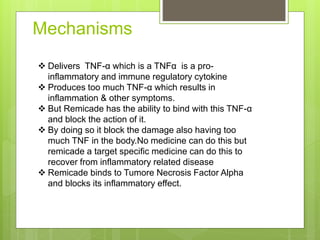
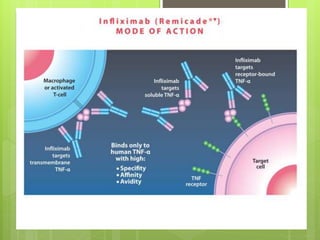
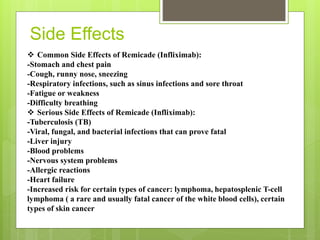
Remicade, also known as Infliximab, is a prescription drug administered via intravenous infusion to treat immune system diseases like Crohn's disease, rheumatoid arthritis, and ulcerative colitis. It works by binding to and blocking tumor necrosis factor alpha, which contributes to inflammation. Common side effects include stomach/chest pain and infections, while serious risks include tuberculosis, cancer, and heart failure. The drug requires careful dosage and administration in a medical setting due to its potential side effects.