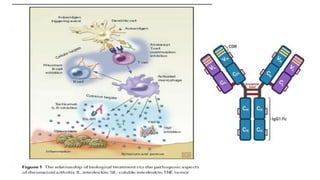
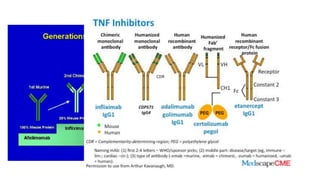
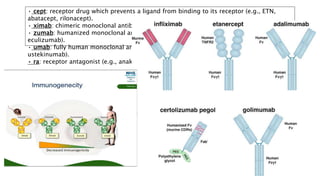
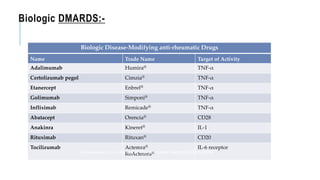
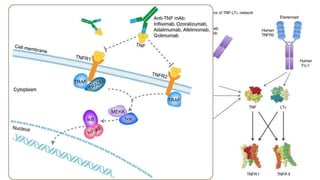
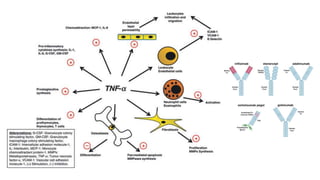
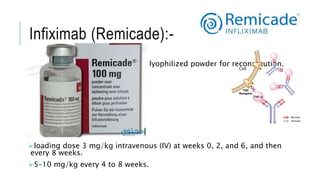
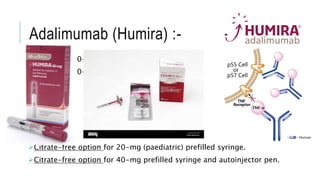
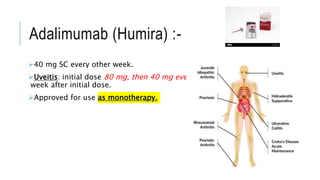
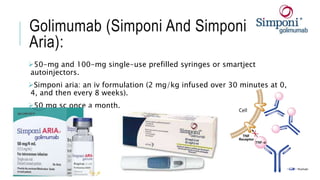
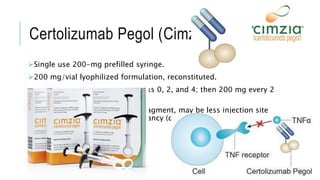
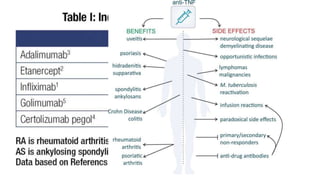
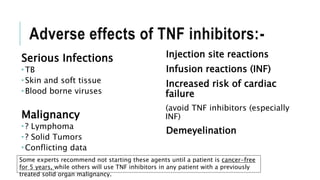
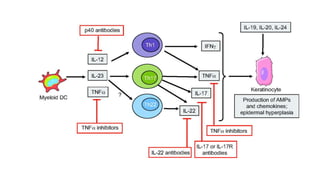
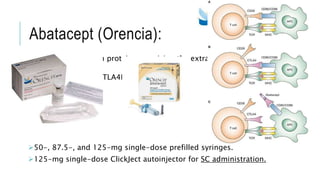
This document provides information on different biological disease-modifying anti-rheumatic drugs (biologic DMARDs) including their names, targets, dosages, administration methods, and potential adverse effects. It discusses TNF inhibitors (infliximab, etanercept, adalimumab, golimumab, certolizumab), non-TNF biologics (abatacept, tocilizumab, rituximab, ustekinumab), and considerations for when to initiate or discontinue biologics. Guidelines are provided on screening requirements, contraindications, and monitoring safety labs when prescribing these agents.