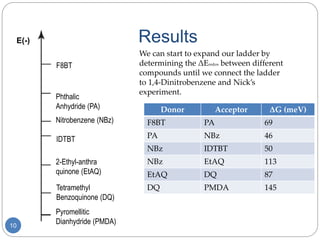
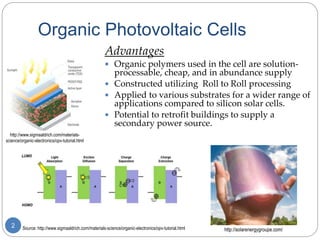
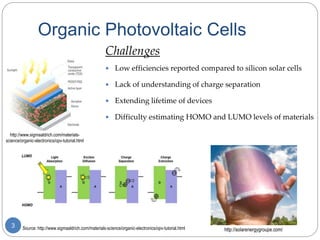
The document describes an experiment to develop a redox ladder of organic polymers and molecules for organic photovoltaic cells by measuring their reduction potentials in THF without electrolyte using pulse radiolysis. The experiment obtained reduction potentials for several donor and acceptor materials and was able to relate their energies to expand the redox ladder. Developing this redox ladder through measurement of reduction potentials could aid in rational design of organic photovoltaic devices.

![5
PCPDTBT
[TBAPF6]
CoCp2 0/+
*Information on the reduction potentials in 0.1M [TBAPF6] in THF was taken from another experiment
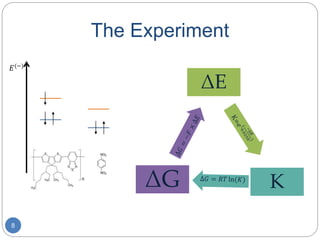
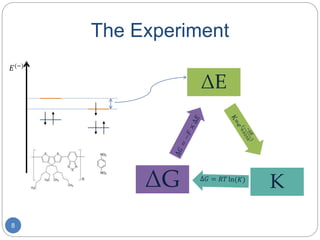
Creating the Ladder
p-NBz](https://image.slidesharecdn.com/343b09de-41f8-49c4-95f1-5869630ffac8-161214162853/85/Redox-Potentials-using-Pulse-Radiolysis-5-320.jpg)
![5
[TBAPF6]
CoCp2 0/+
p-NBz
My experiment: Fill in the redox
ladder from 1,4-Dinitrobenzene
(p-NBz) to the solvated electron.
Nick’s experiment:
Test the fit function against
Cobaltocenium (CoCp2) and
p-NBz, while determining the
oxidation/reduction values.
*Information on the reduction potentials in 0.1M [TBAPF6] in THF was taken from another experiment
Creating the Ladder
PCPDTBT](https://image.slidesharecdn.com/343b09de-41f8-49c4-95f1-5869630ffac8-161214162853/85/Redox-Potentials-using-Pulse-Radiolysis-6-320.jpg)
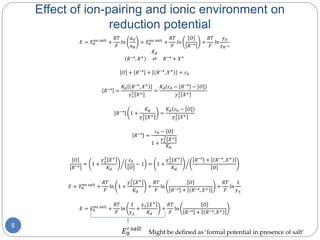
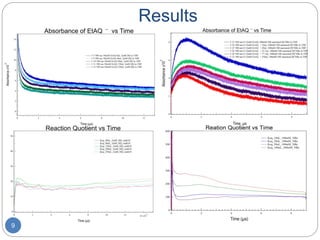
![Methods
7
0.5cm
Acceptor
Donor
Electron pulse
Light source
Filter
Detector
𝐷−∙
+ A ↔ D + 𝐴−∙
𝐾 =
𝐷 [𝐴−∙]𝛾 𝐴−∙
𝐴 [𝐷−∙]𝛾 𝐷−∙
𝐴 = 𝜀𝐶 𝑜 𝐿
𝐶 𝑜 = 𝐷−∙ + [𝐴−∙]
[𝐴−∙]
𝐷−∙
=
A 𝐷 − A 𝐷+𝐴
A 𝐷+𝐴](https://image.slidesharecdn.com/343b09de-41f8-49c4-95f1-5869630ffac8-161214162853/85/Redox-Potentials-using-Pulse-Radiolysis-8-320.jpg)