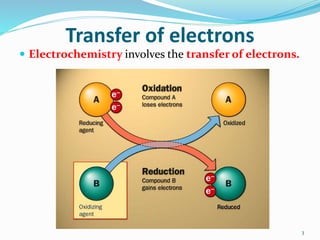
(1) Electrochemistry involves the transfer of electrons during chemical reactions and electrical changes brought about by chemical changes.
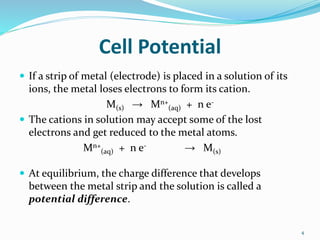
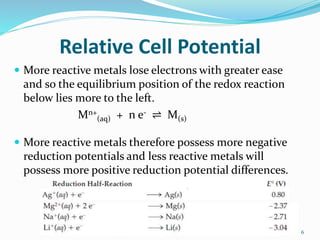
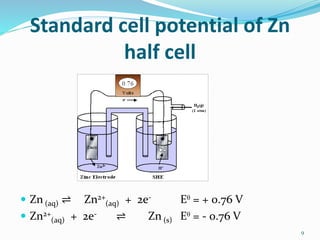
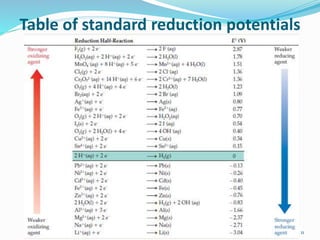
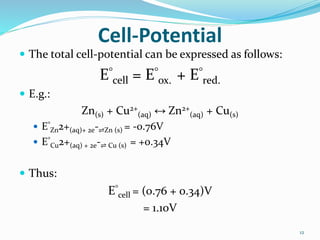
(2) Cell potential, measured in Volts, is the tendency of a species to lose or gain electrons compared to the Standard Hydrogen Electrode potential of 0.00V.
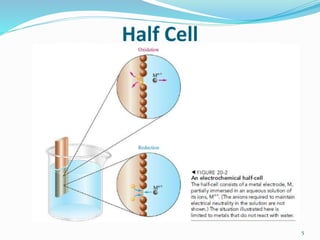
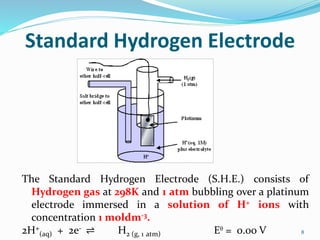
(3) The Standard Hydrogen Electrode consists of hydrogen gas bubbling over a platinum electrode in a solution of 1M hydrogen ions, and its reduction potential is defined as 0.00V.