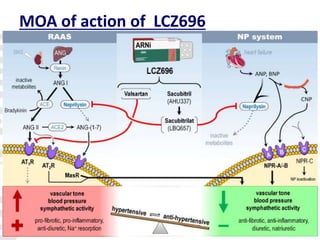
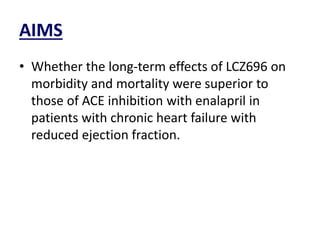
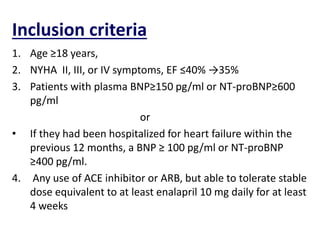
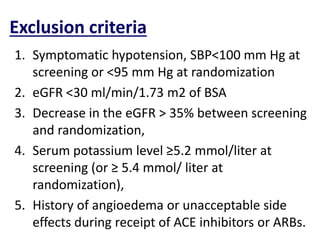
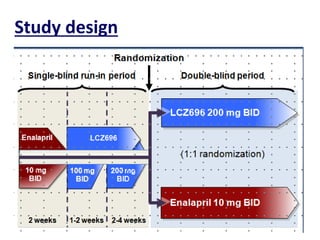
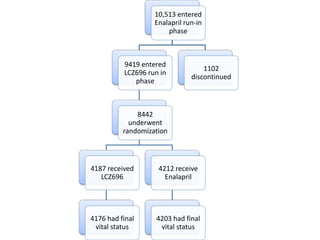
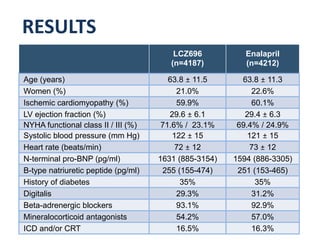
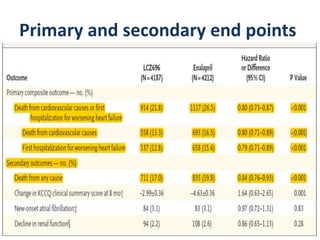
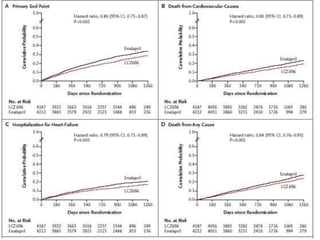
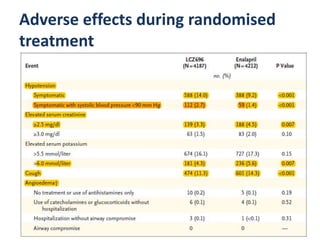
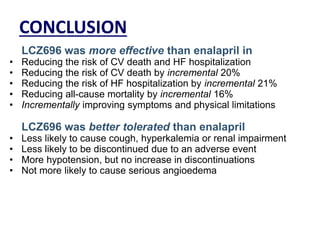
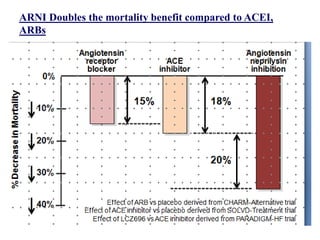
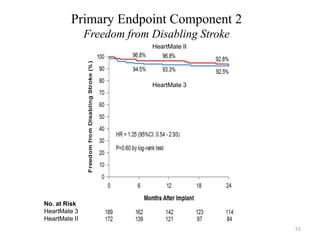
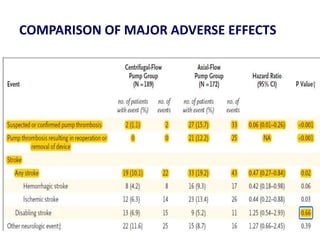
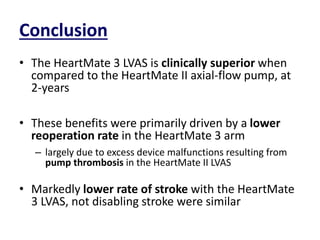
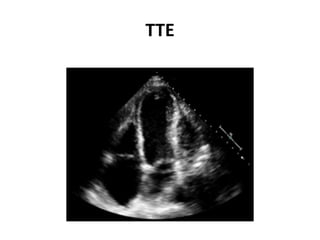
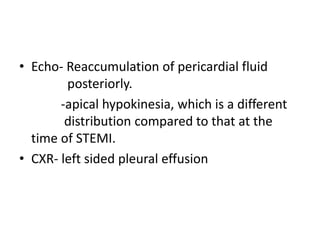
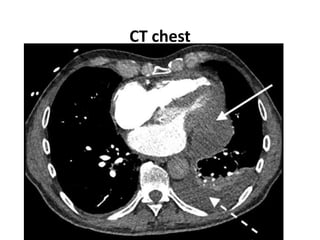
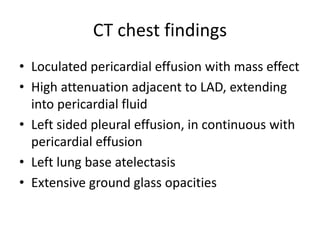
- The PARADIGM-HF trial found that treatment with LCZ696 (a combination of sacubitril and valsartan) was more effective at reducing cardiovascular death and heart failure hospitalization compared to enalapril. LCZ696 also reduced overall mortality more than enalapril.
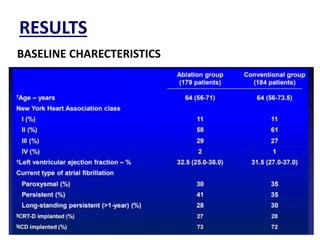
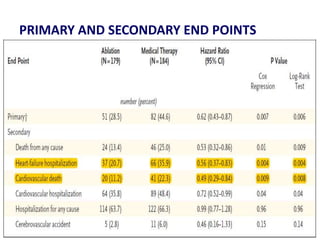
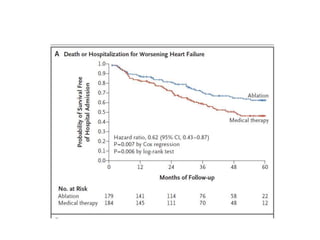
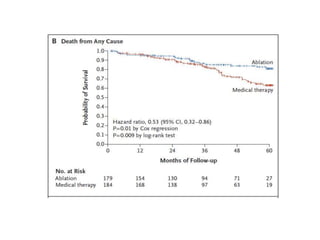
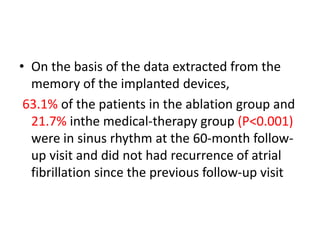
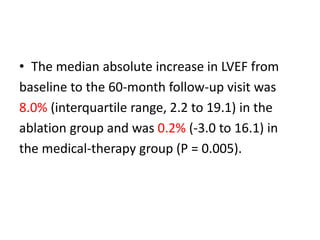
- The CASTLE-AF trial found that catheter ablation for atrial fibrillation was superior to pharmacological rate or rhythm control methods for reducing mortality and heart failure hospitalization in patients with left ventricular dysfunction and atrial fibrillation. Ablation resulted in more time in sinus rhythm and a greater increase in left ventricular ejection fraction.
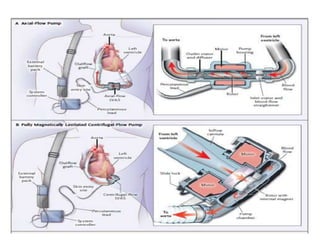
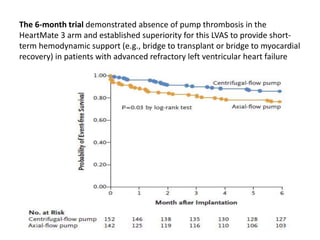
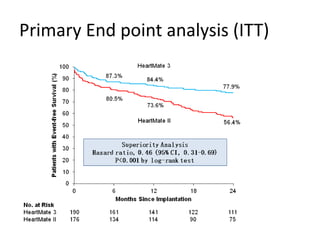
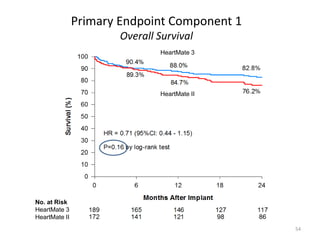
- The document reviewed results from major clinical trials investigating treatments for heart failure