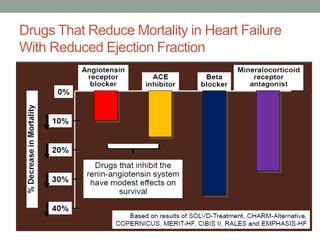
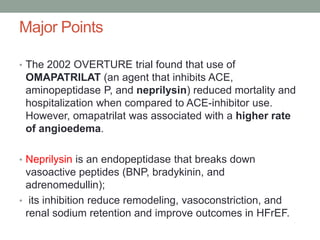
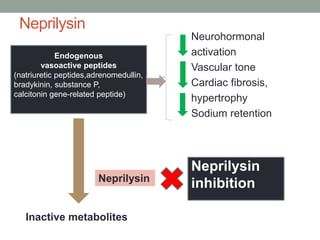
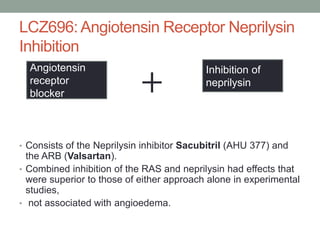
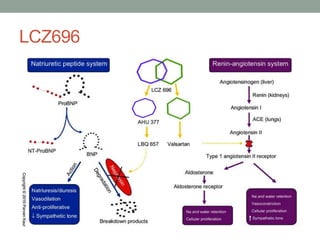
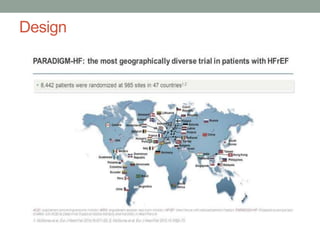
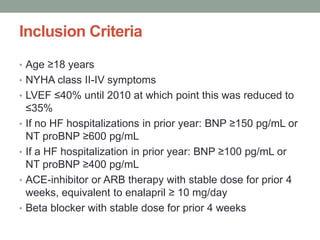
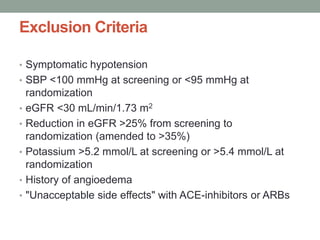
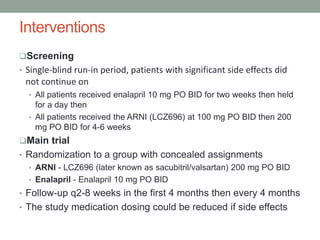
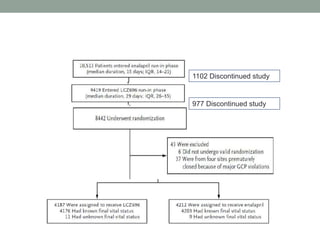
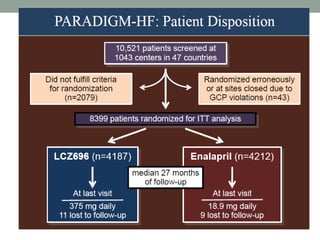
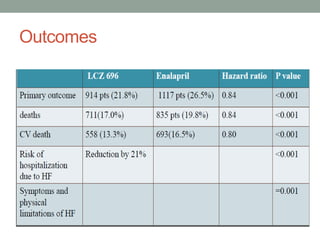
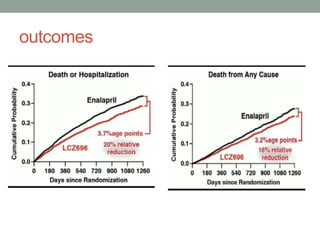
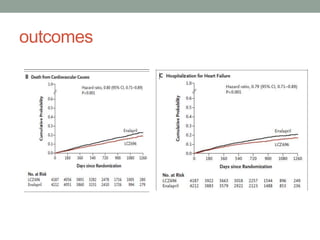
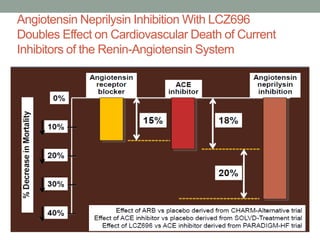
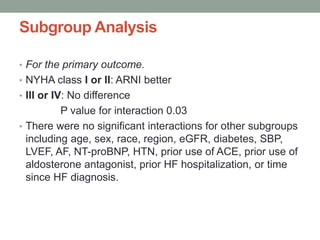
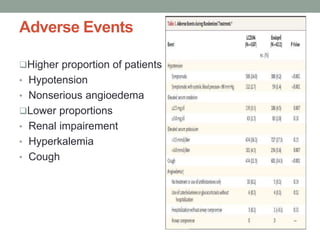
The PARADIGM-HF trial compared the angiotensin receptor-neprilysin inhibitor sacubitril/valsartan to the ACE inhibitor enalapril in patients with heart failure with reduced ejection fraction. It found that sacubitril/valsartan reduced cardiovascular mortality and heart failure hospitalizations compared to enalapril, as well as reducing overall mortality. The trial established sacubitril/valsartan as a new standard of care for treating HFrEF.




![PARADIGM HF Trial
Prospective Comparison of ARNI [Angiotensin Receptor -
Neprilysin Inhibitor] with ACEI [Angiotensin Converting
Enzyme Inhibitor] to Determine Impact on Global Mortality
and Morbidity in Heart Failure Trial.
ANGIOTENSIN Receptor –NEPRILYSIN Inhibition
versus
ENALAPRIL in Heart Failure](https://image.slidesharecdn.com/paradigmheartfailuretrial-200623042523/85/Paradigm-HF-trial-6-320.jpg)