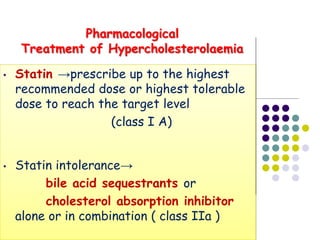
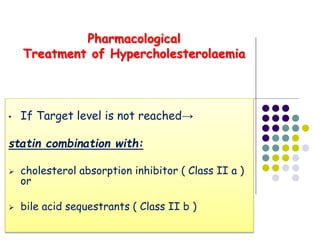
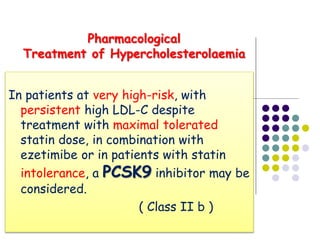
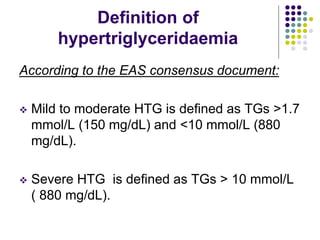
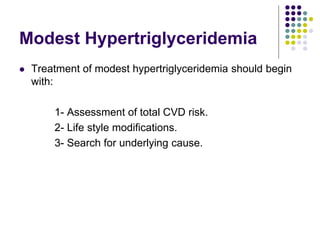
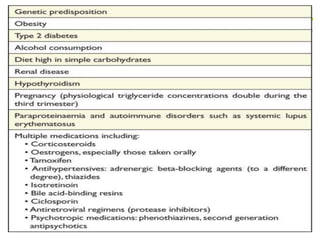
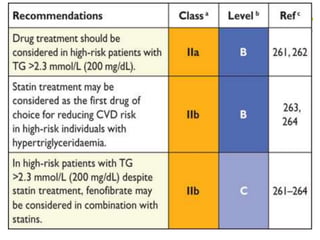
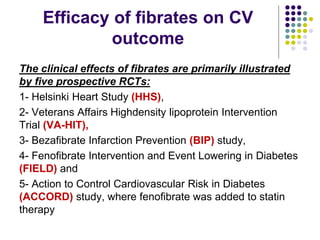
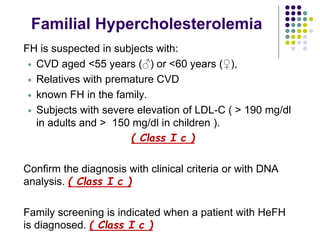
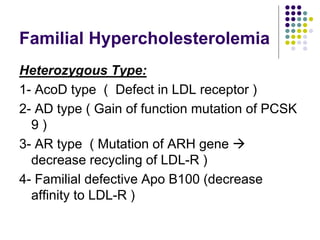
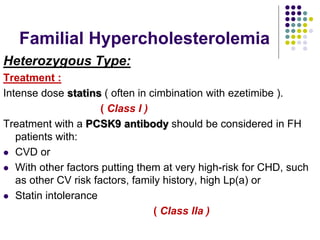
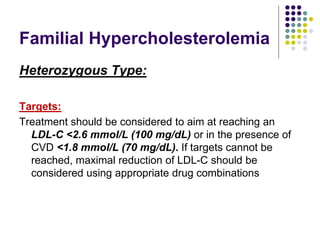
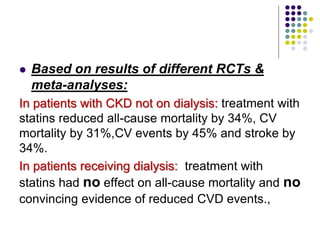
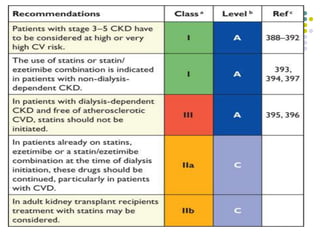
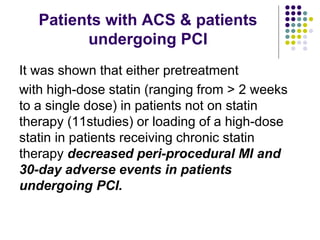
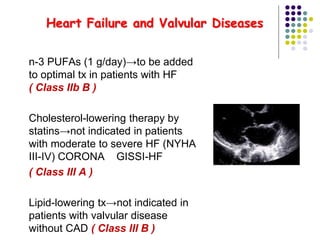
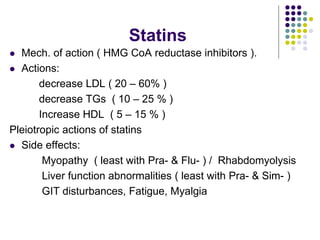
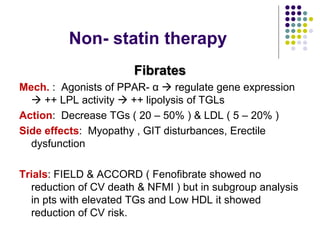
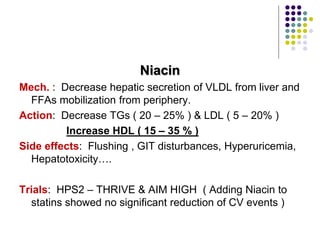
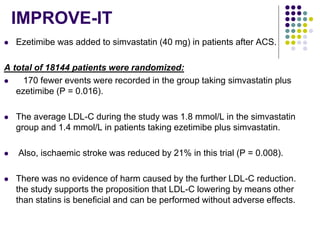
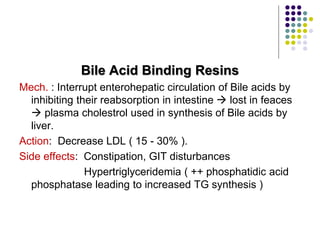
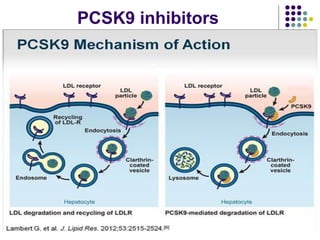
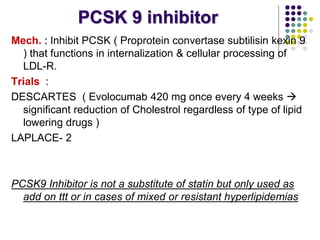
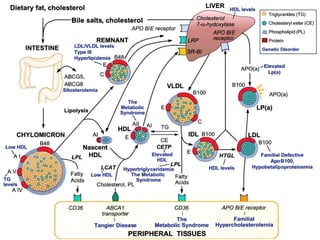
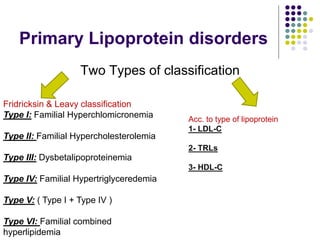
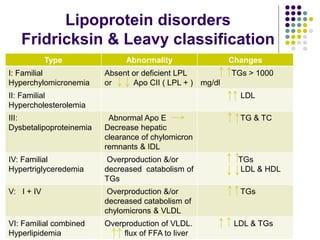
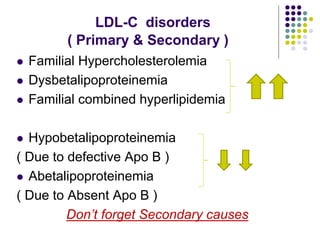
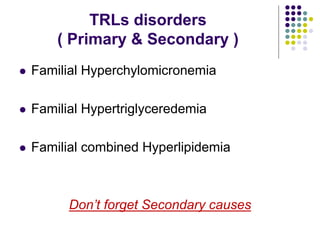
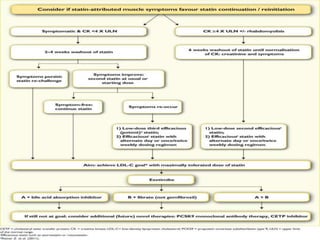
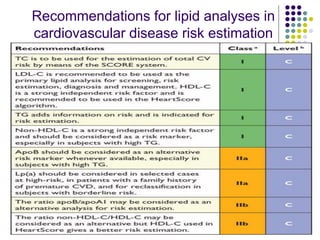
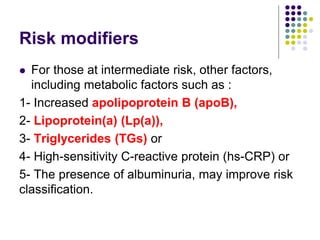
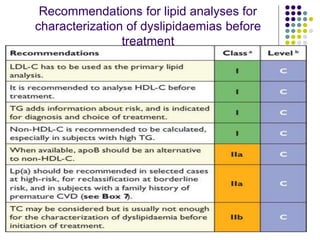
This document provides guidelines for the management of dyslipidemia from the European Society of Cardiology in 2016. It discusses lipid profiling, total cardiovascular risk assessment, treatment strategies, lifestyle modifications, treatment targets, and choice of treatment. Lipid profiling is recommended for those with cardiovascular disease, at increased risk, or for risk stratification. LDL-C is the primary treatment target, while non-HDL-C and apoB are secondary targets. Lifestyle changes and statin therapy are first-line treatment, with fibrates, nicotinic acid or PCSK9 inhibitors as options for additional lowering of lipids. Guidelines for treatment targets and special populations are also covered.



![Non-HDL cholesterol
Non-HDL-C is used as an estimation of the total number
of atherogenic particles in plasma [VLDL +
intermediate-density lipoprotein (IDL) + LDL] .
Non-HDL-C correlates relates well to apo B levels.
Non-HDL-C is easily calculated from TC minus HDL-C.](https://image.slidesharecdn.com/dyslipidemianew-161111114020/85/Dyslipidemia-guidelines-8-320.jpg)