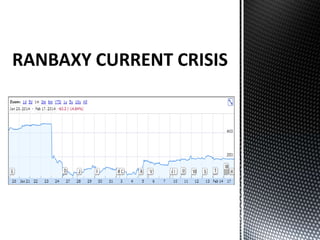
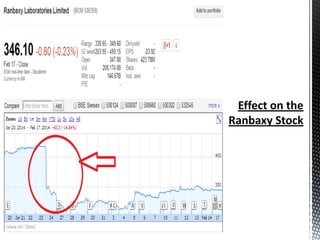
Ranbaxy Laboratories was founded in 1937 in India and became a public company in 1973. It was acquired by Daiichi Sankyo in 2008. Whistleblowers in 2004 revealed that Ranbaxy had fabricated drug test reports, resulting in warnings from the FDA and import bans. Ranbaxy pleaded guilty in 2013 to drug safety violations and paid $500 million in fines. Several plants including Toansa were banned by the FDA for quality control issues such as contaminated products, poor facilities, and inaccurate data. The culture change needed to resolve Ranbaxy's problems fully will take strong leadership over the long term.