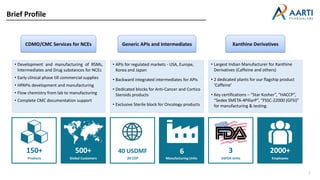
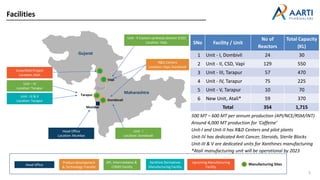
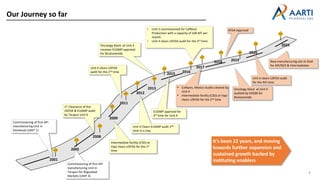
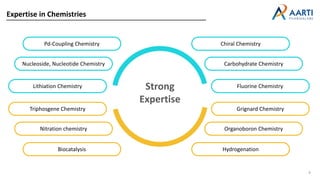
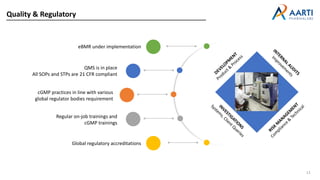
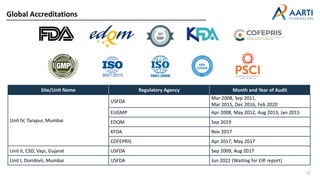
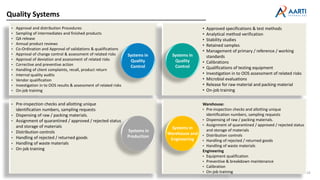
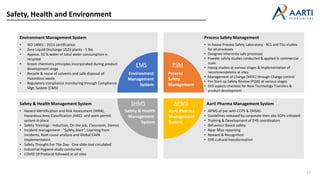
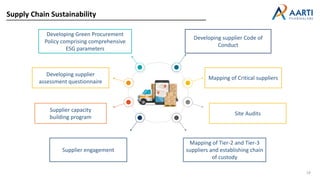
Aarti Pharma Labs is a global pharmaceutical manufacturer with over 500 customers worldwide, specializing in APIs, intermediates, and xanthine derivatives. They have multiple manufacturing units in India and significant certifications ensuring compliance with global regulatory standards, while emphasizing their expertise in high-potency APIs and flexible manufacturing processes. The company aims for sustainable growth and continuous development of new products while ensuring quality and safety across their operations.