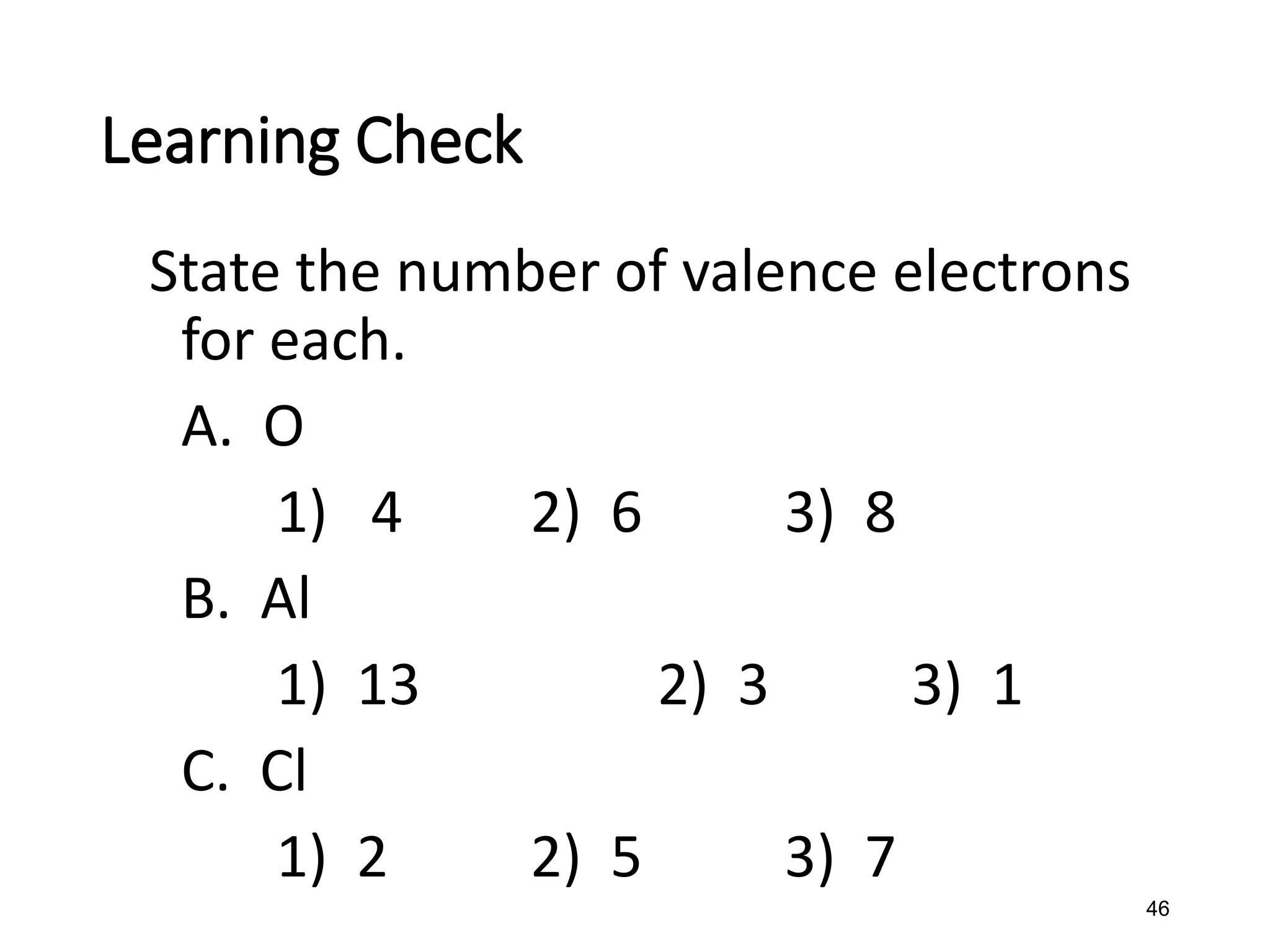
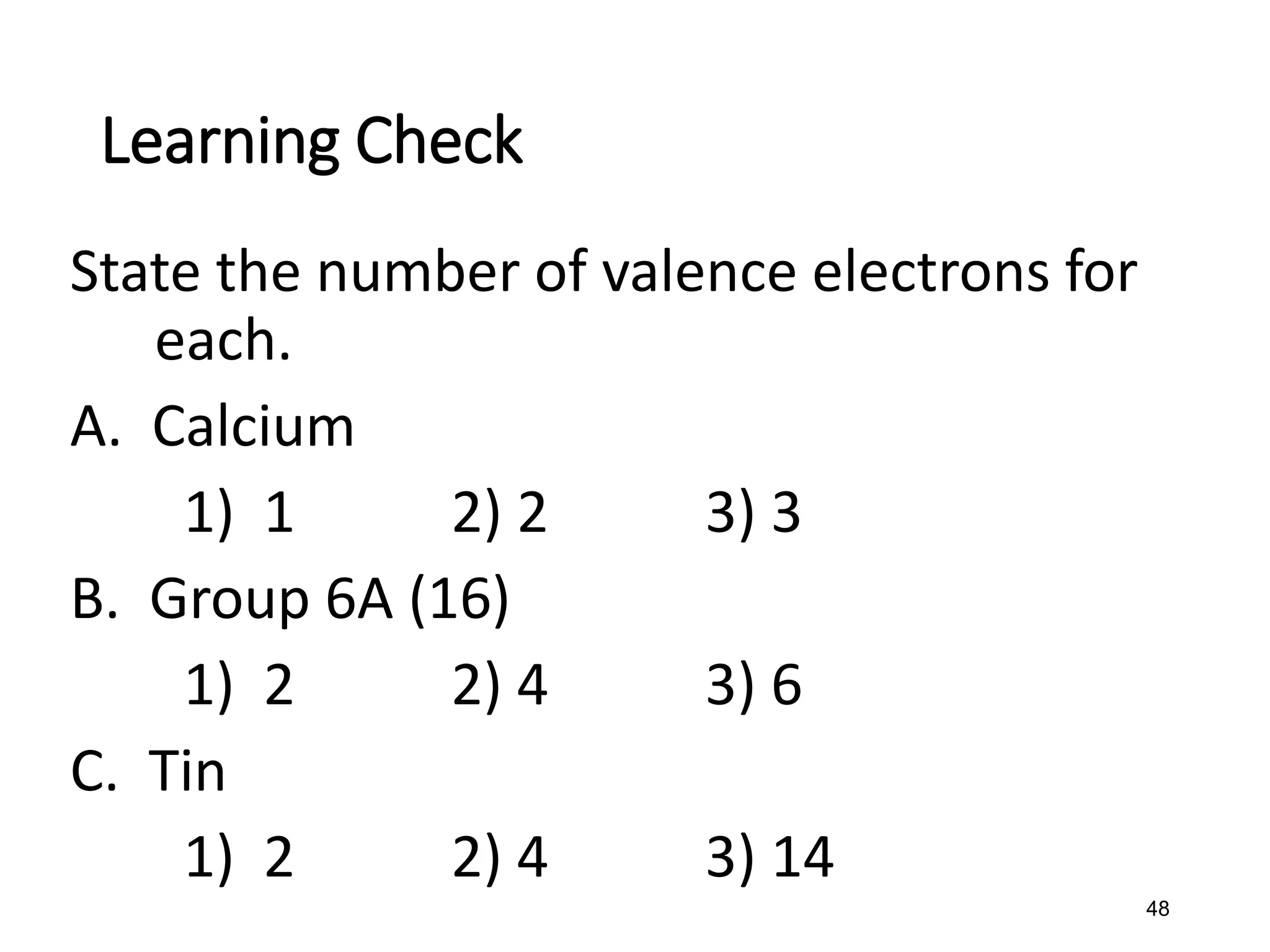
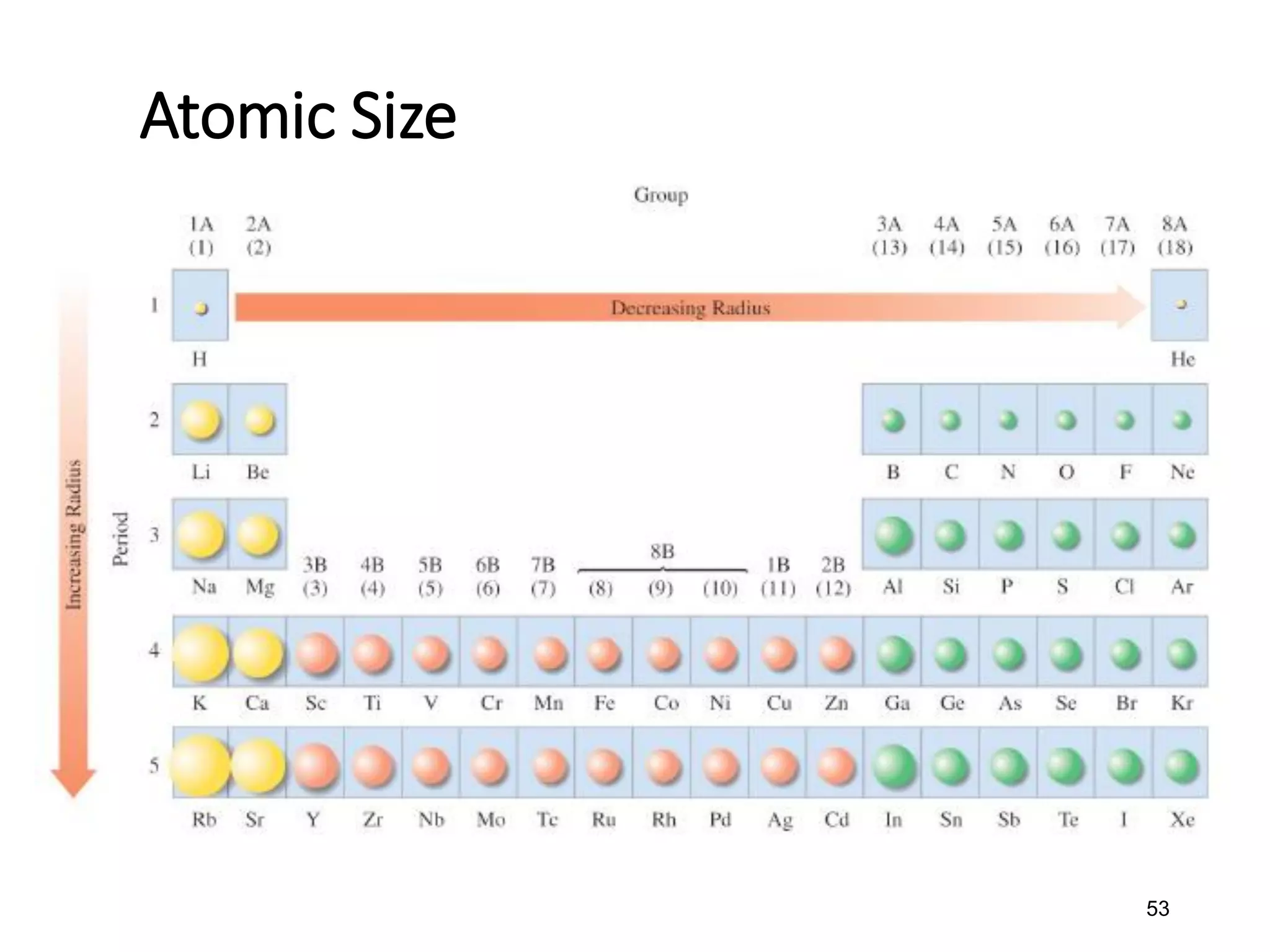
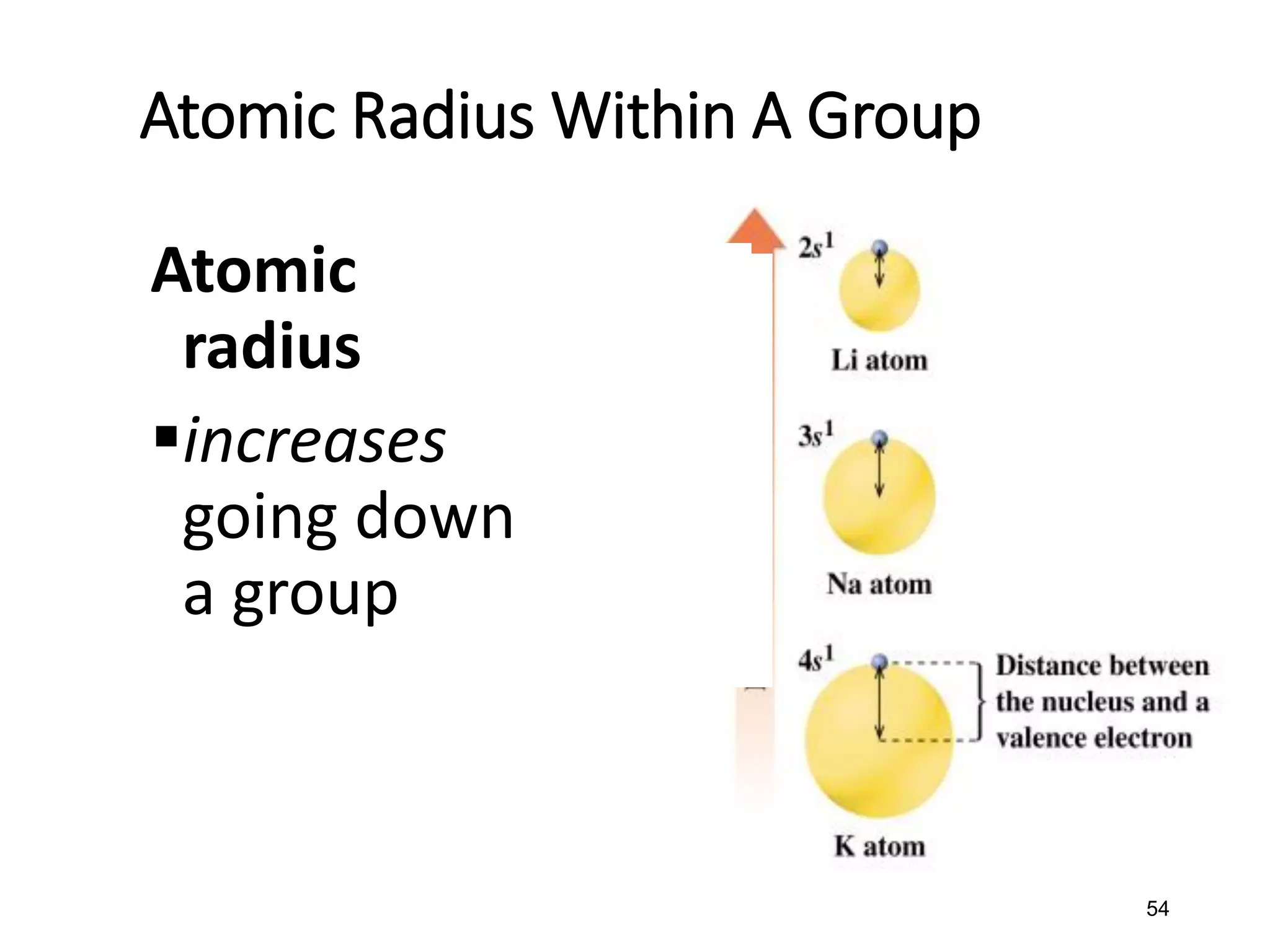
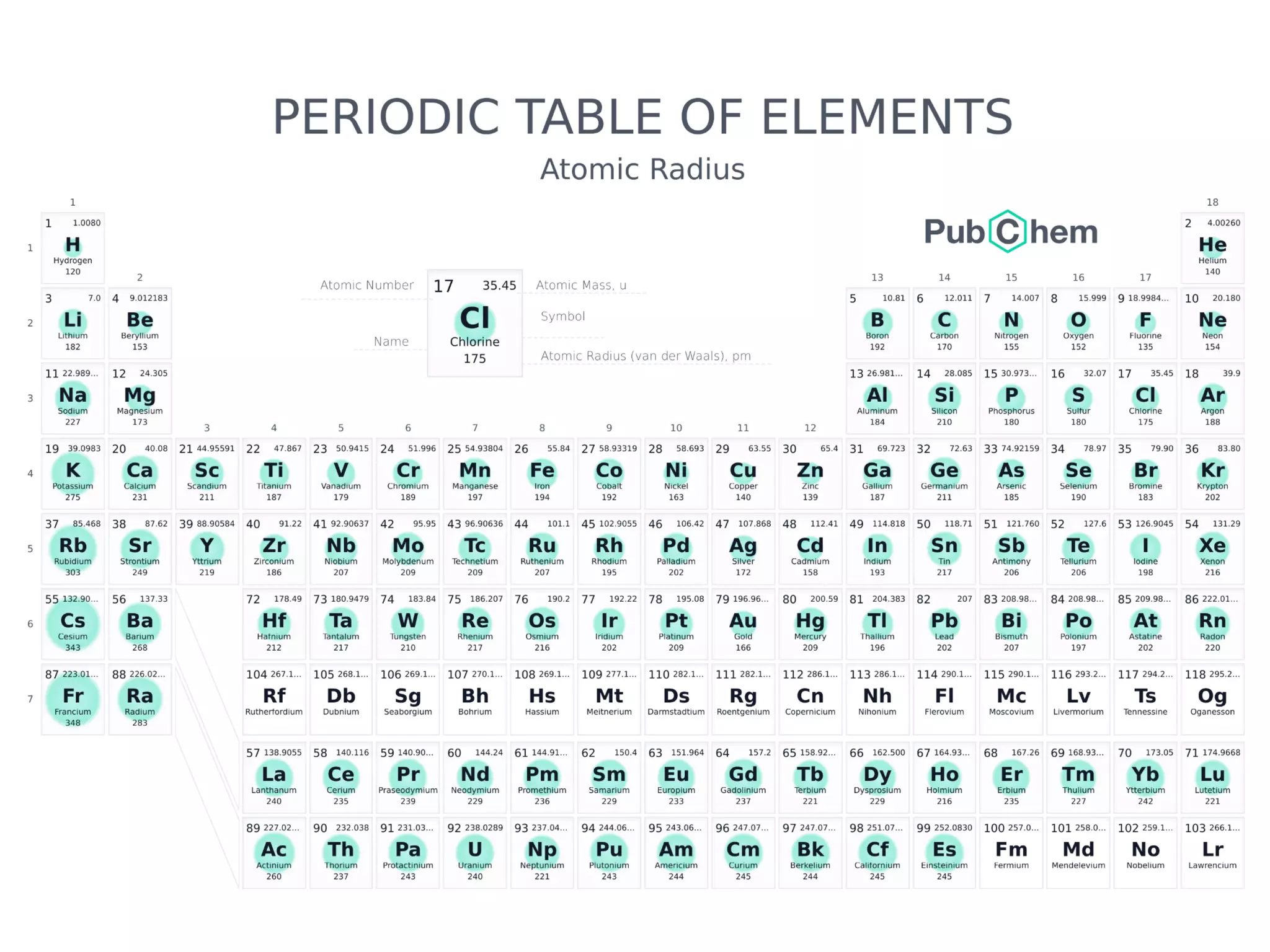
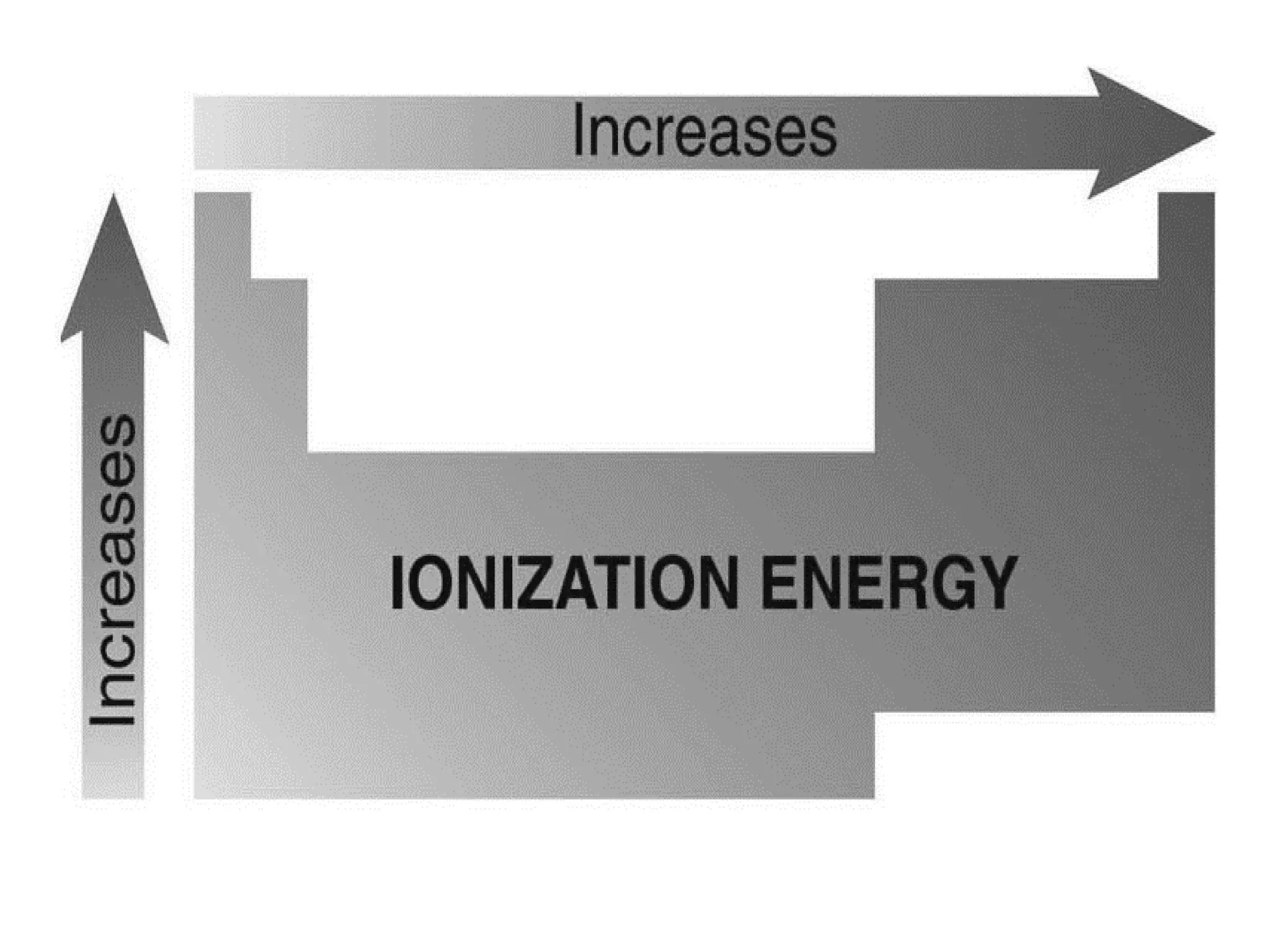
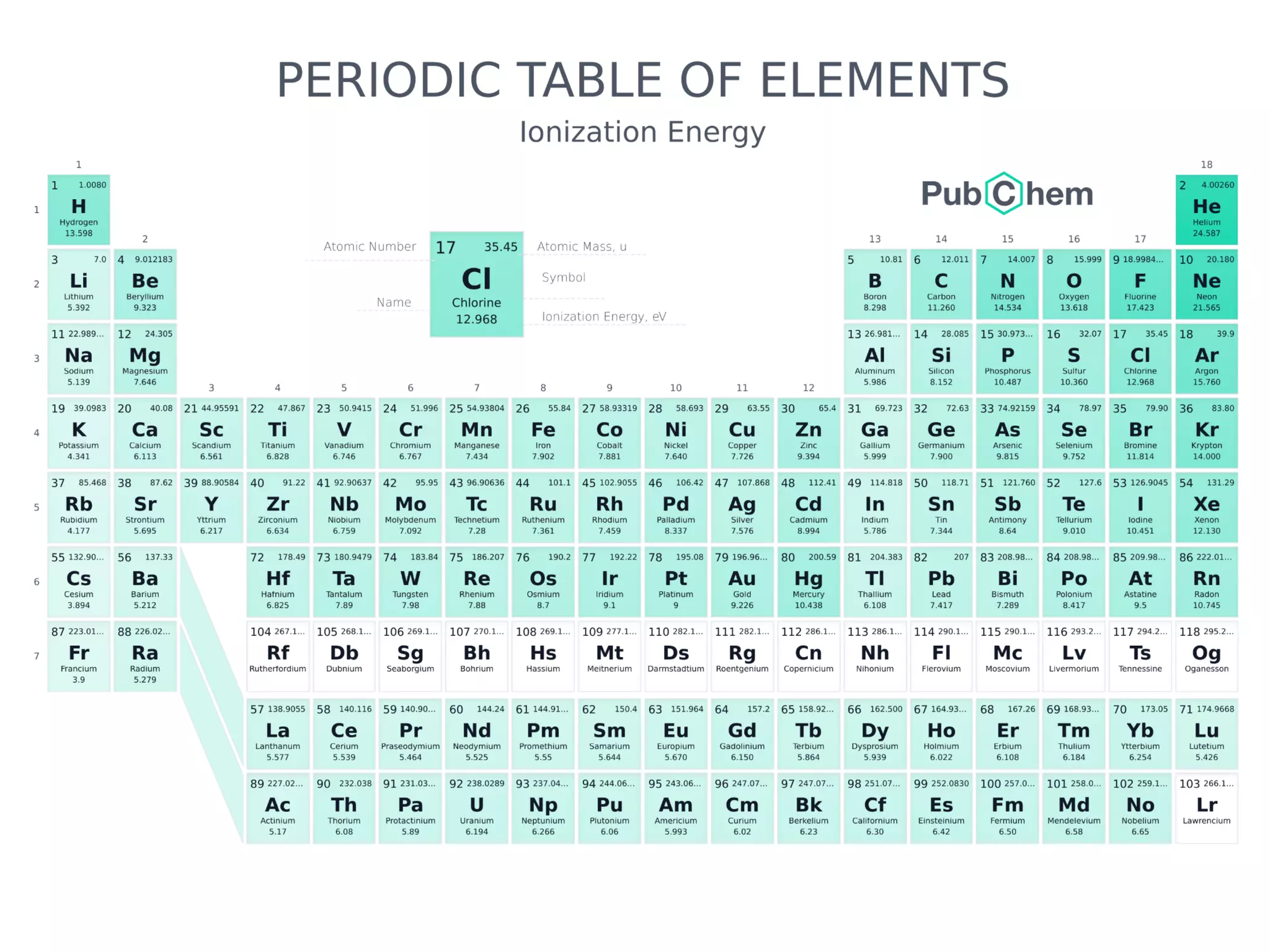
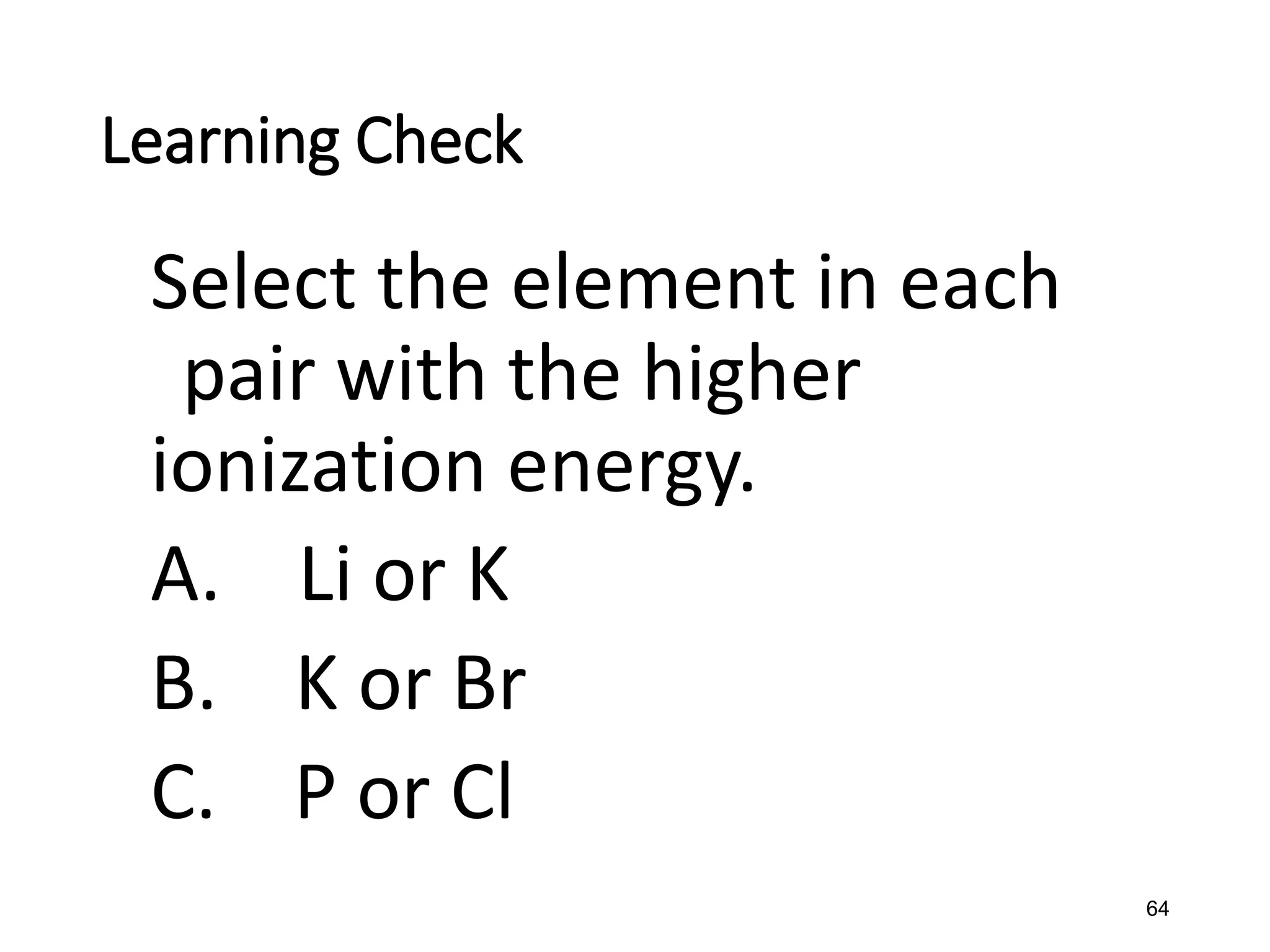
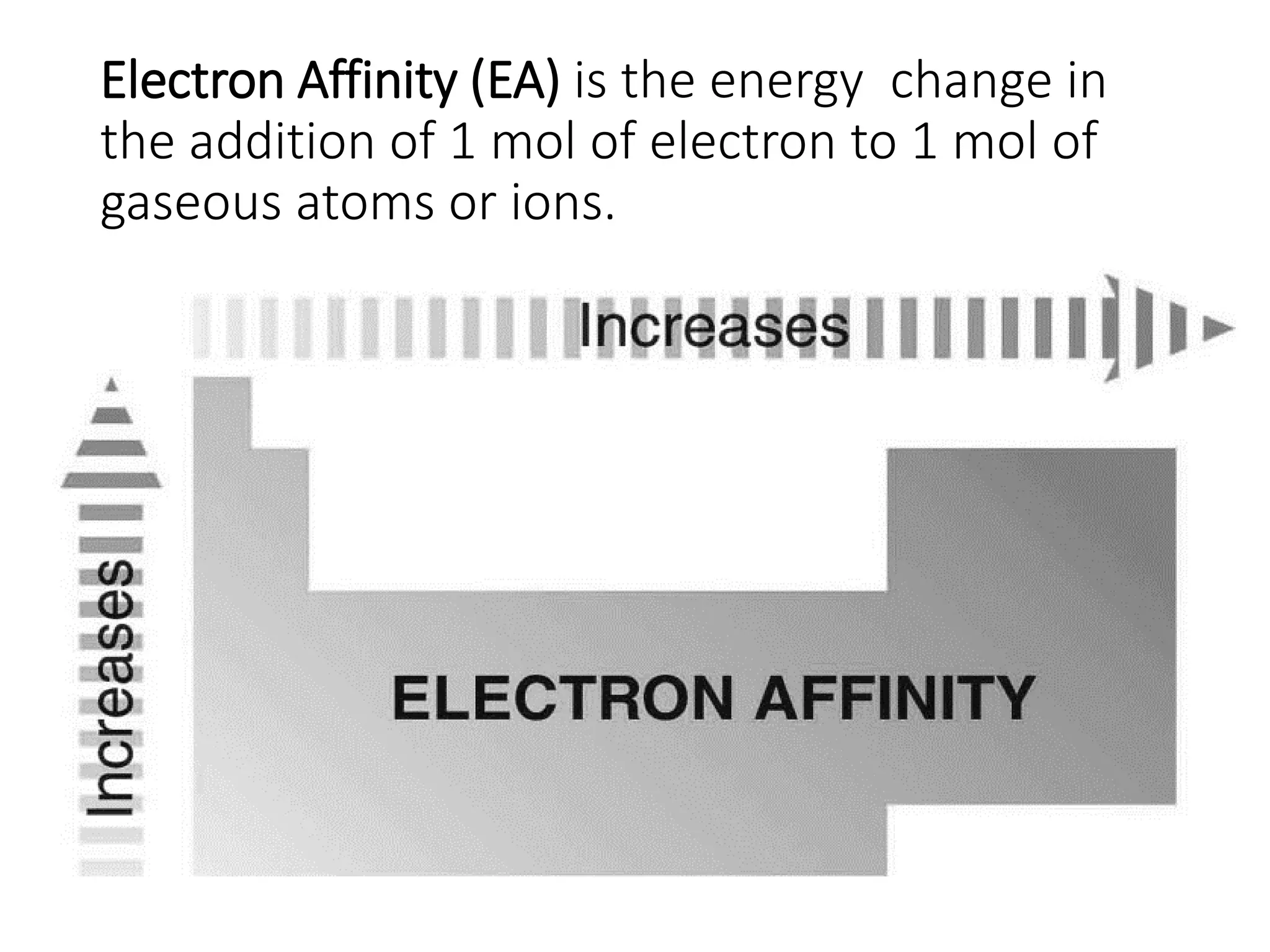
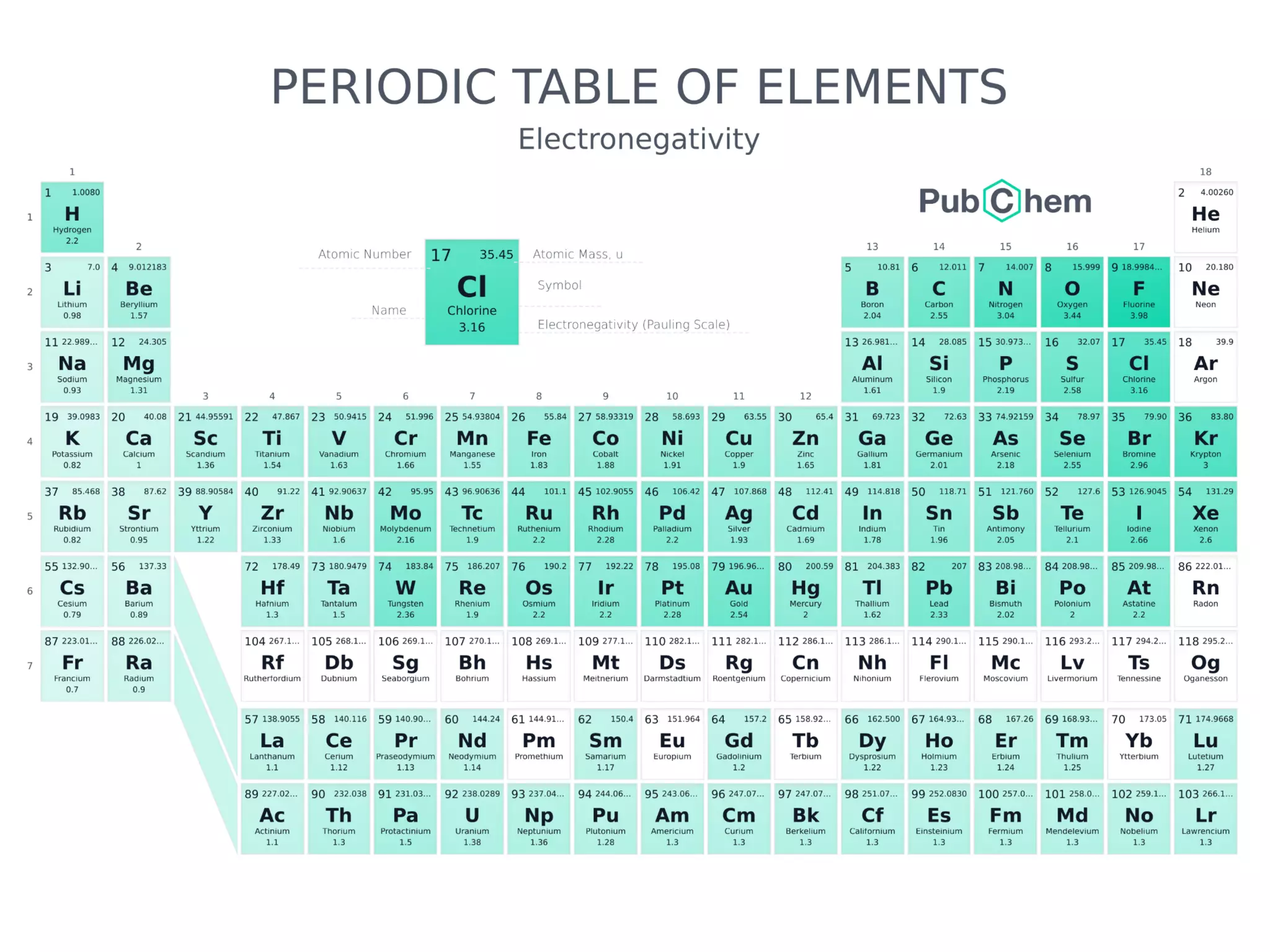
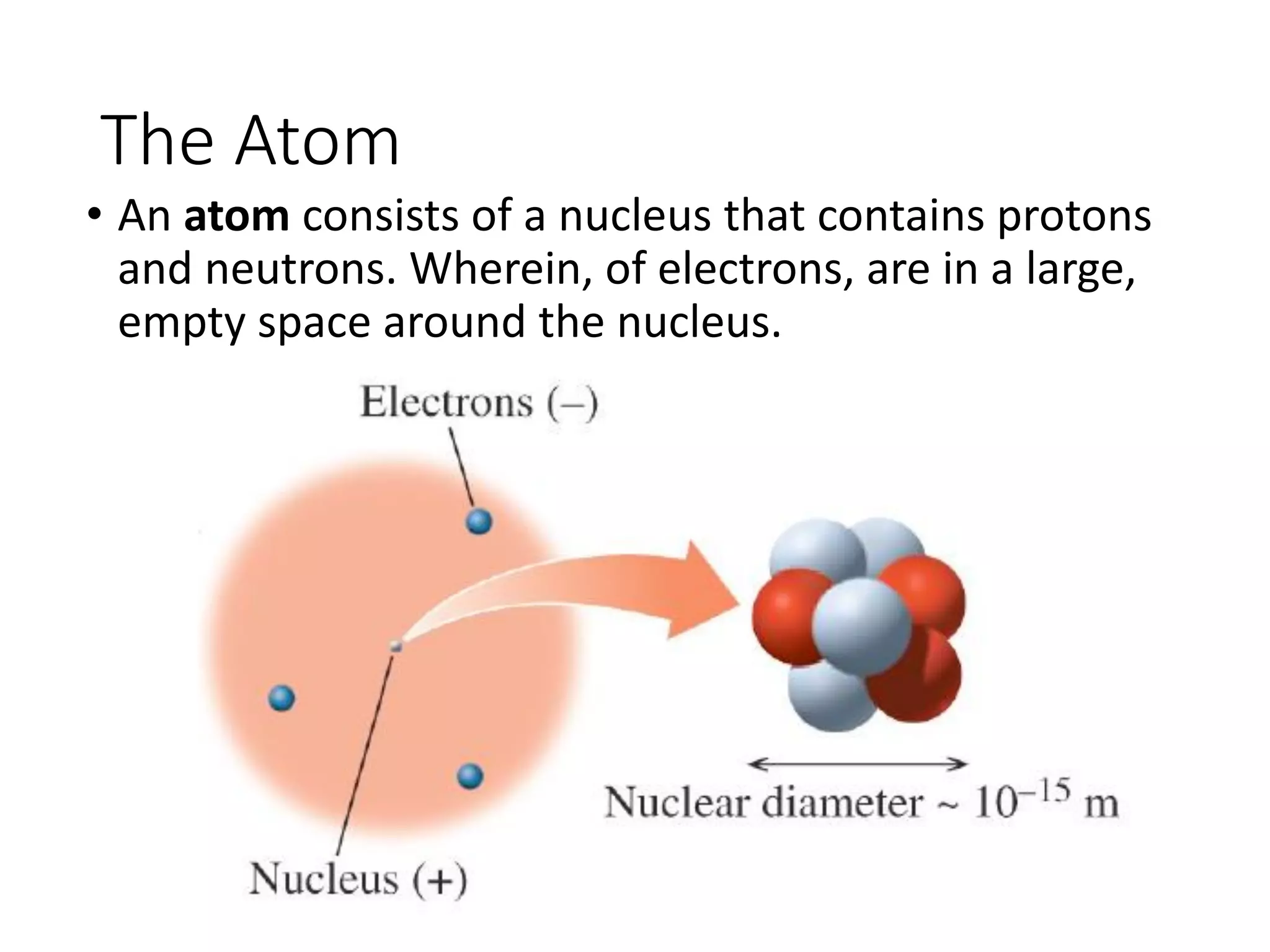
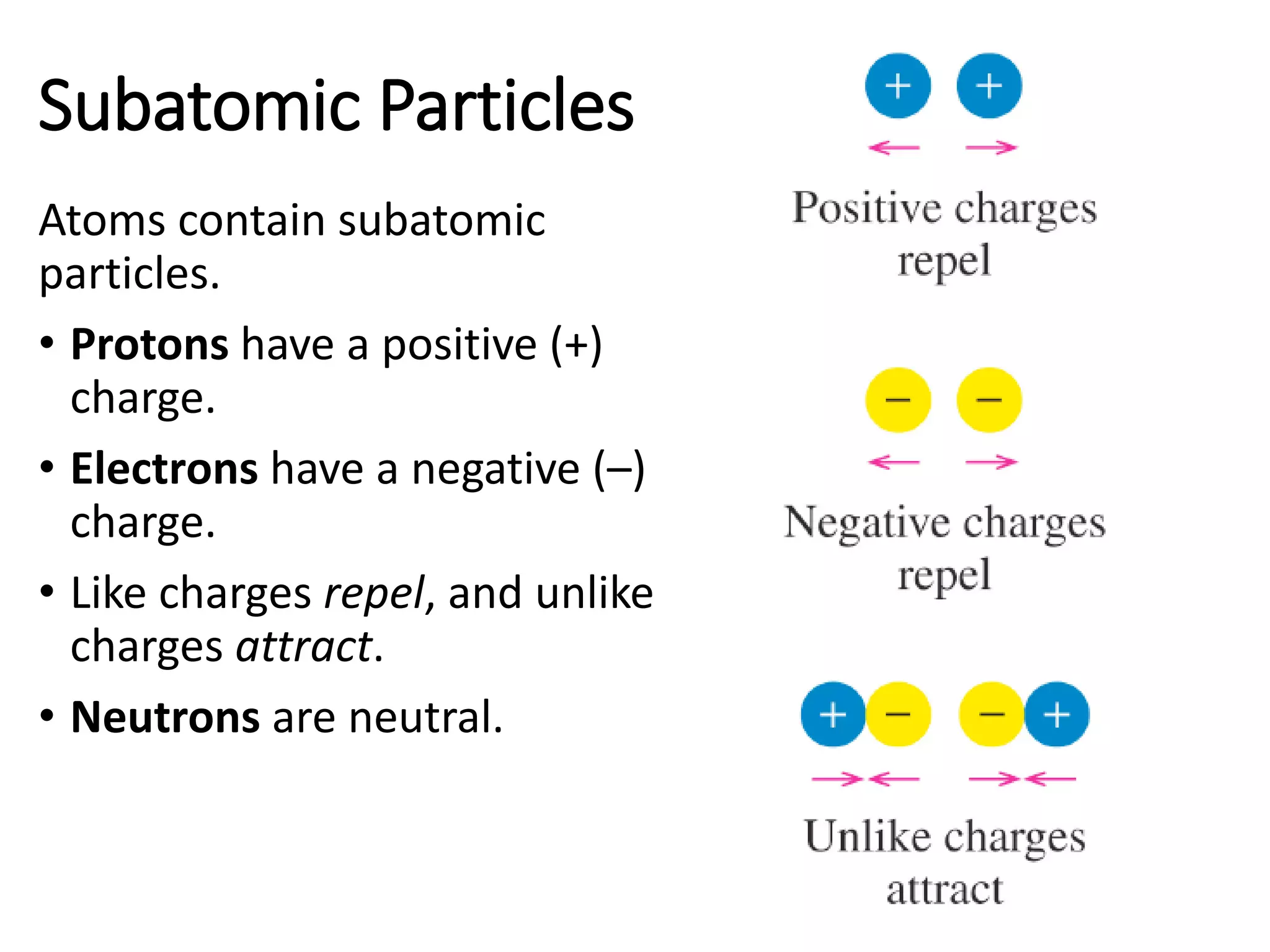
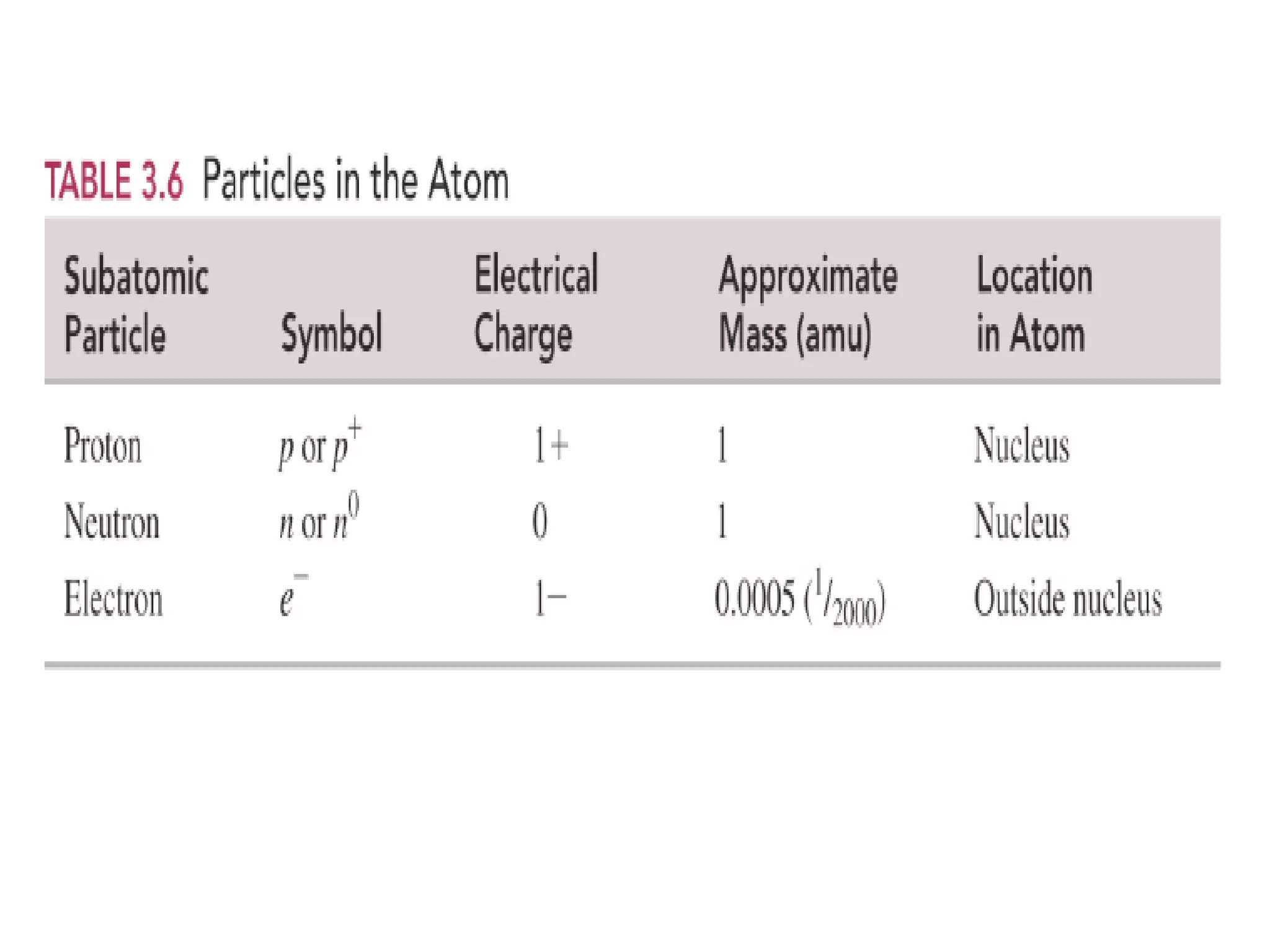
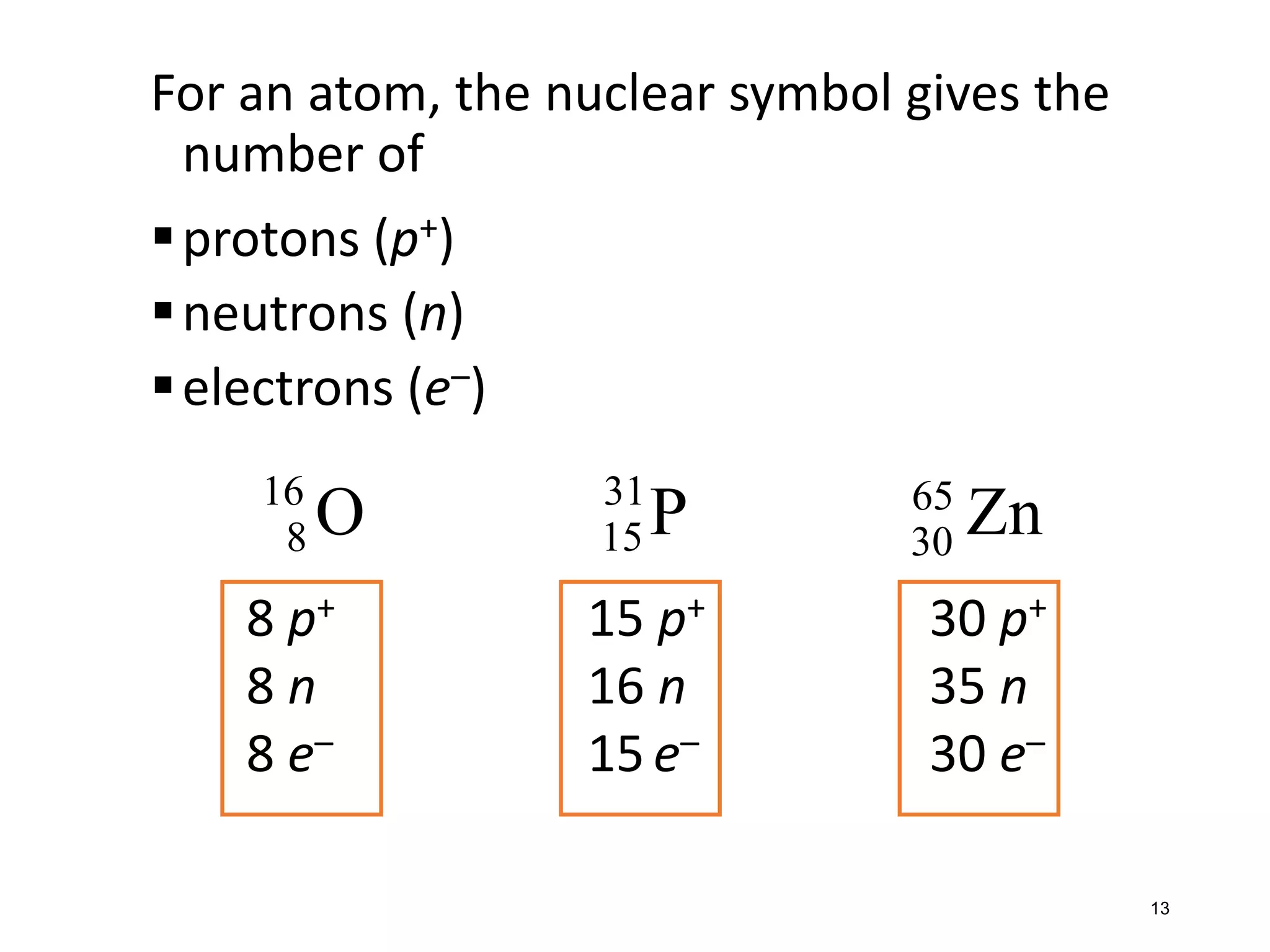
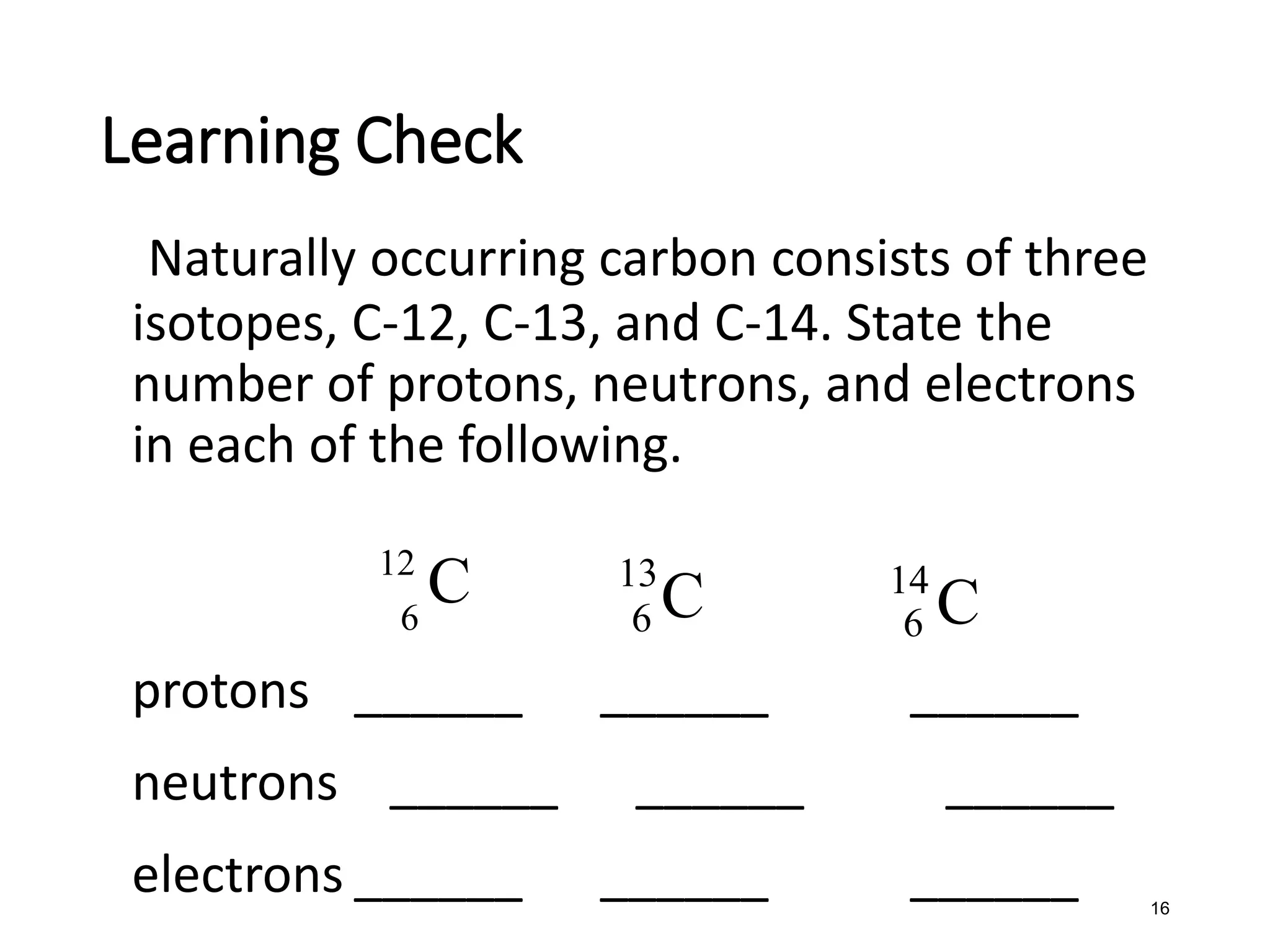
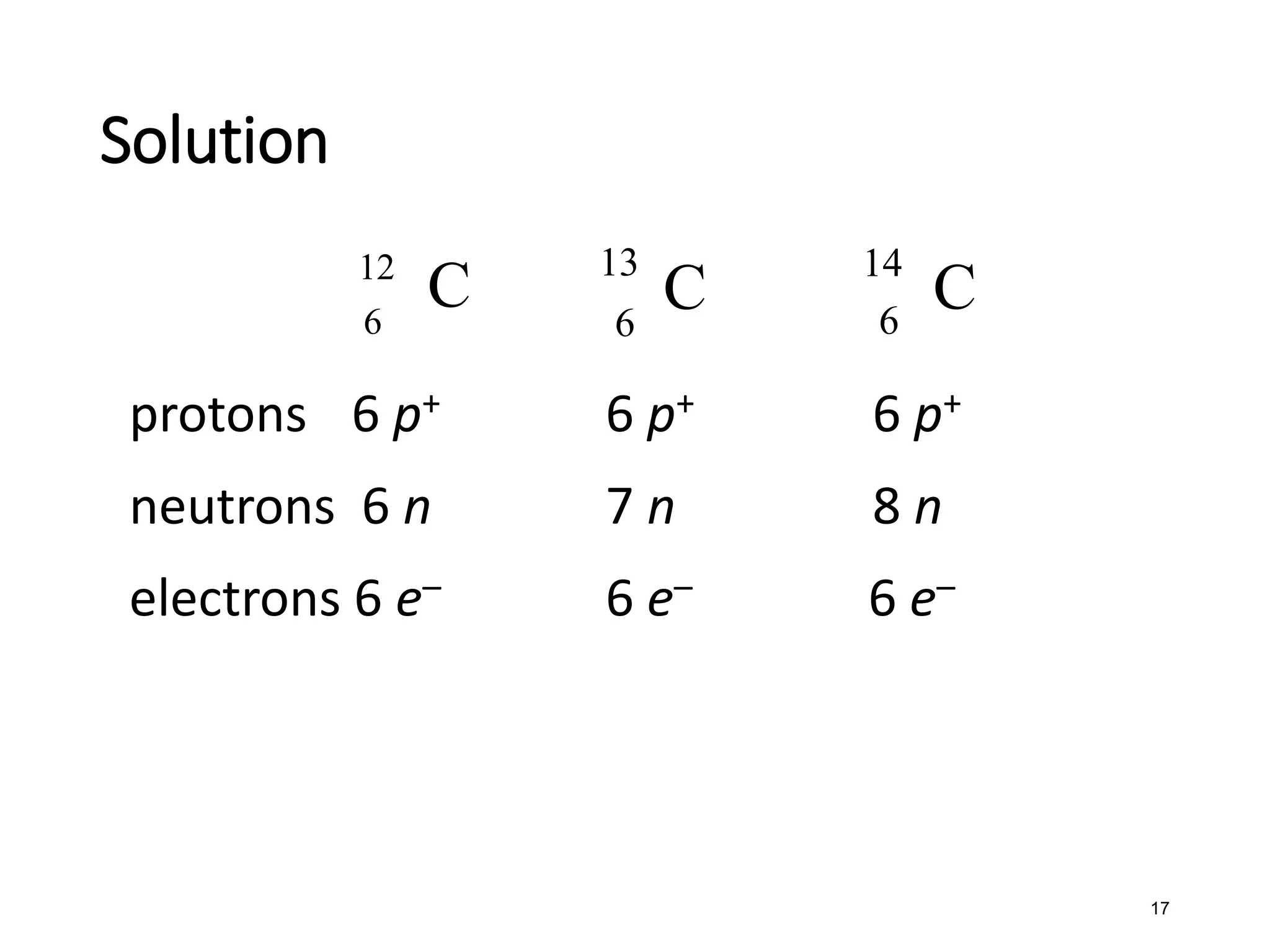
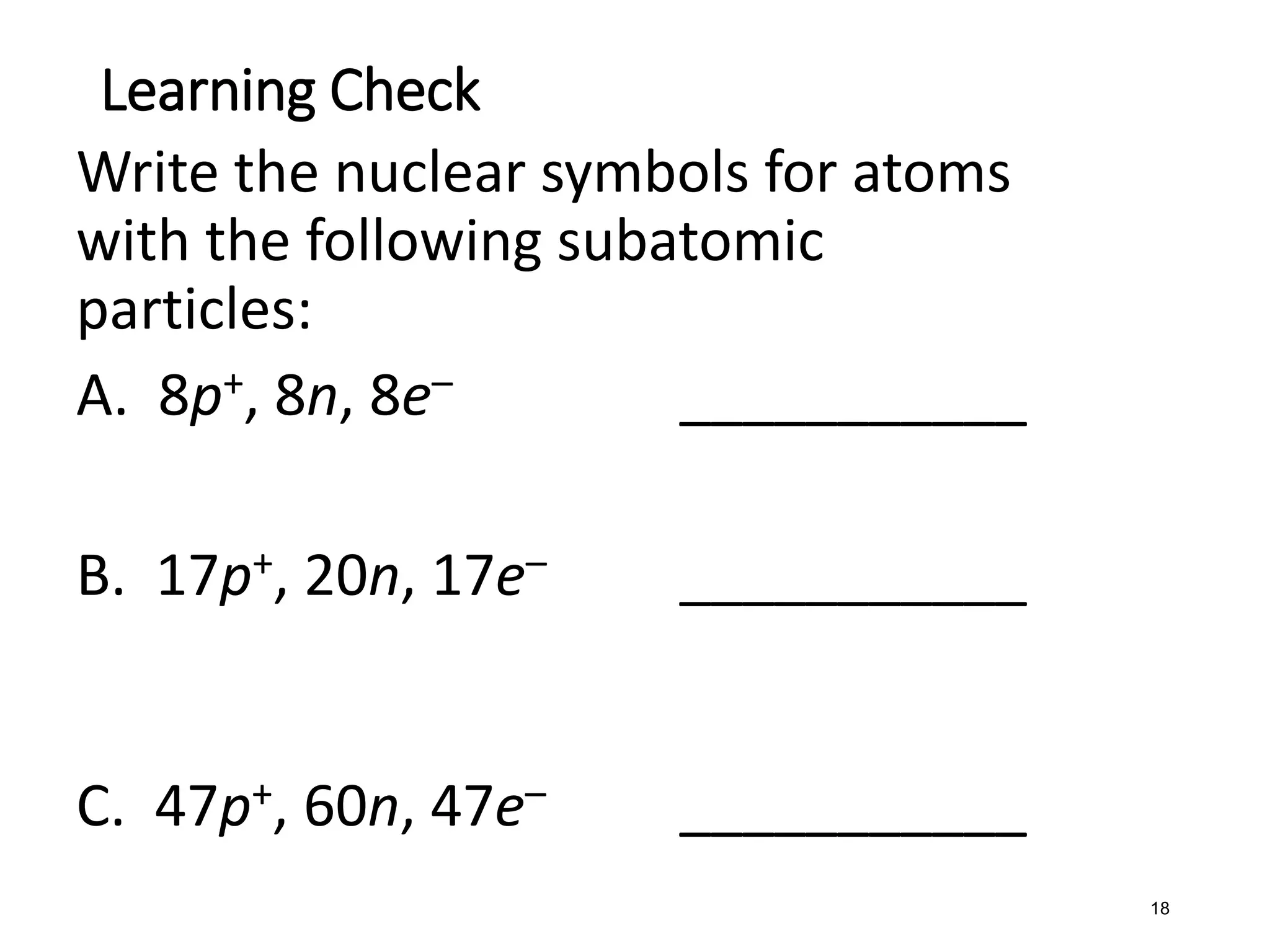
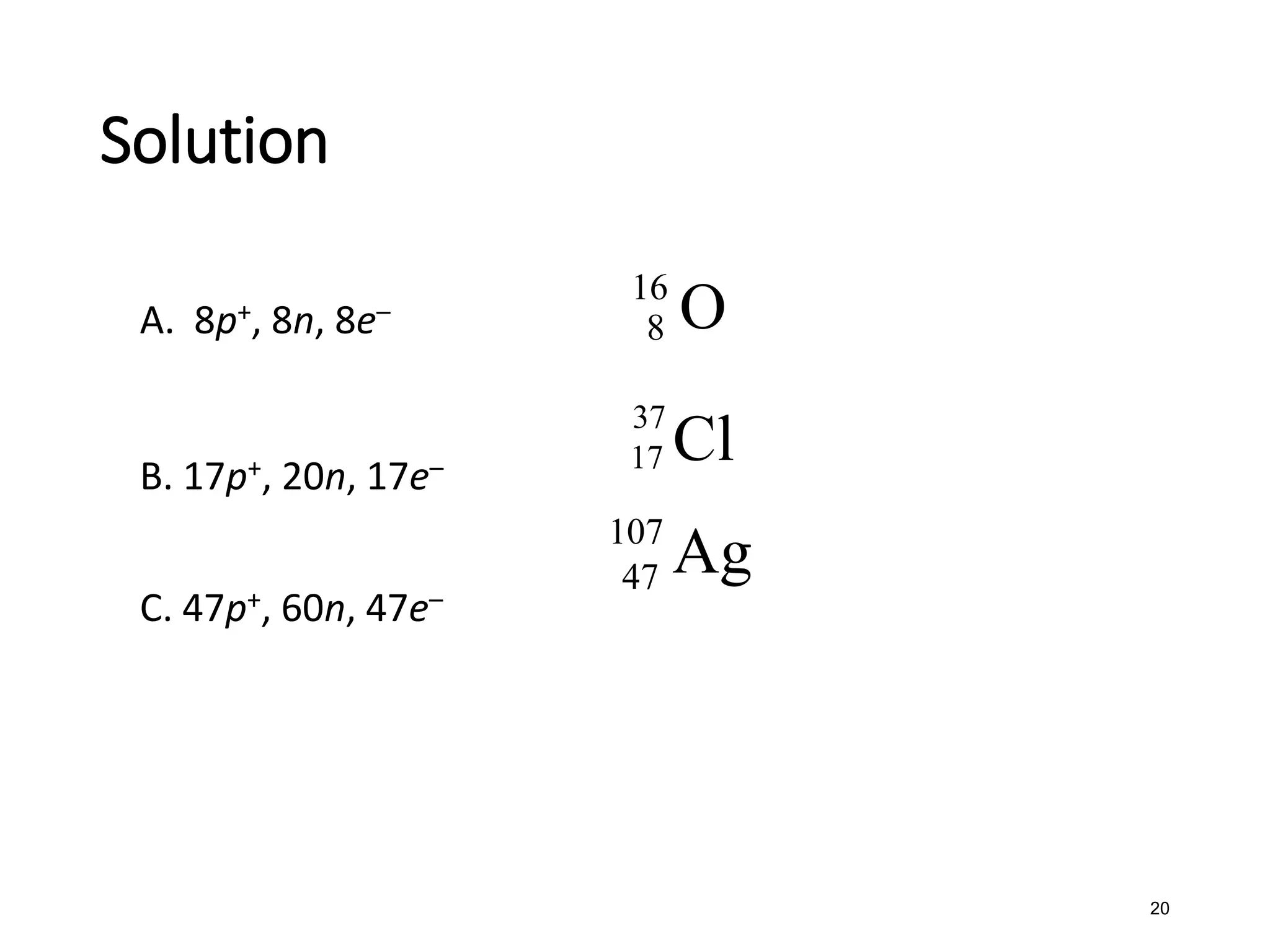
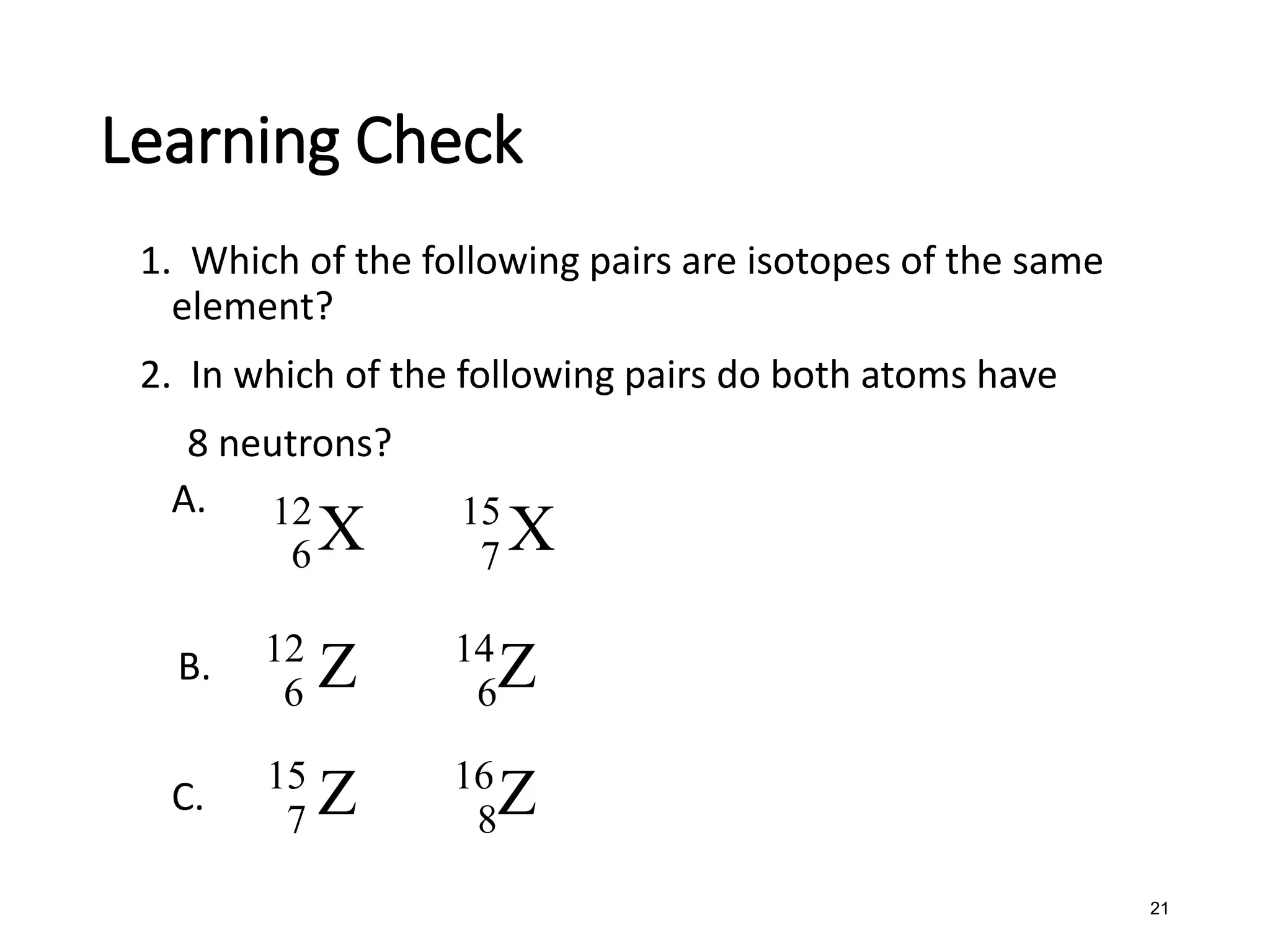
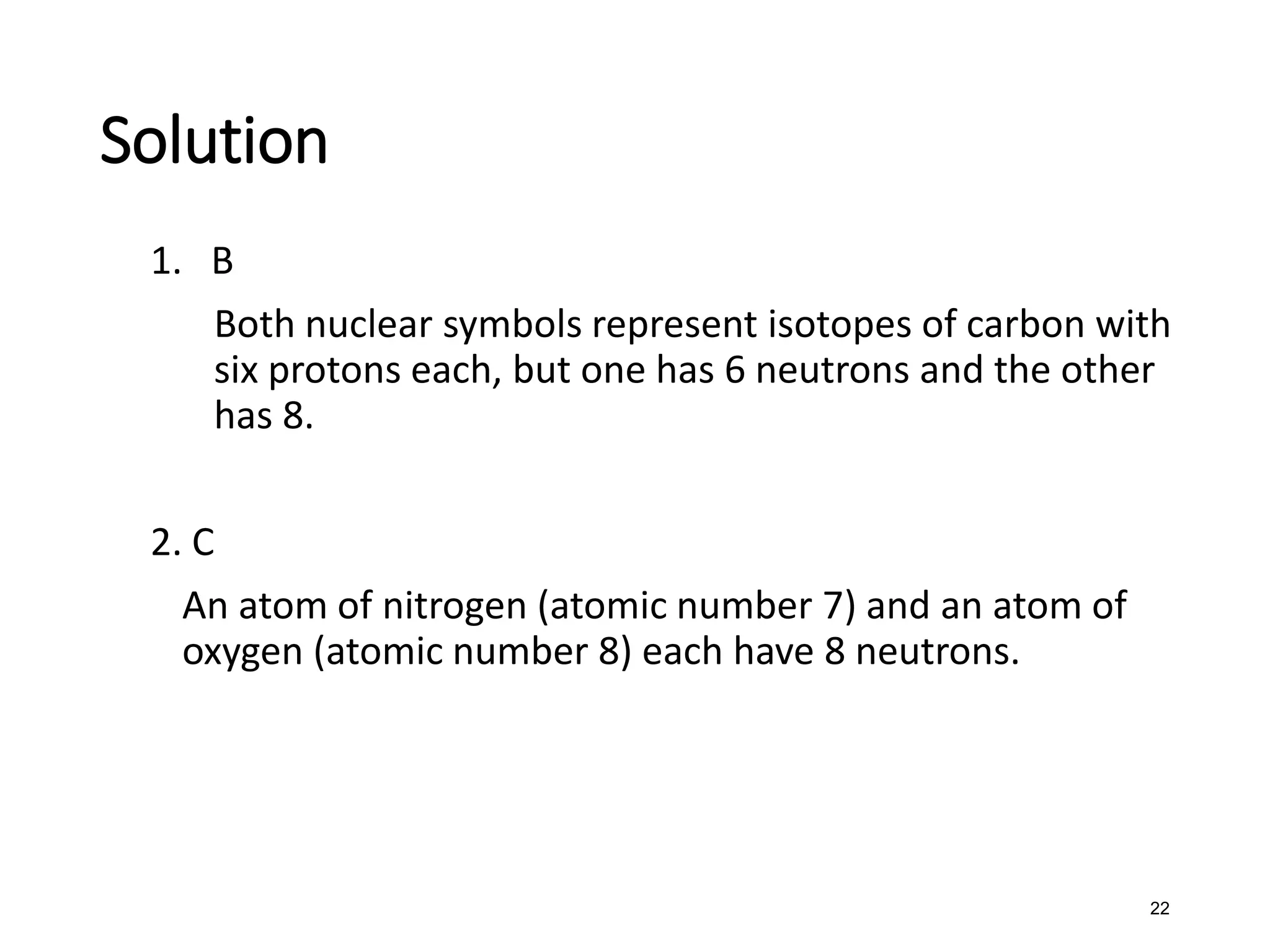
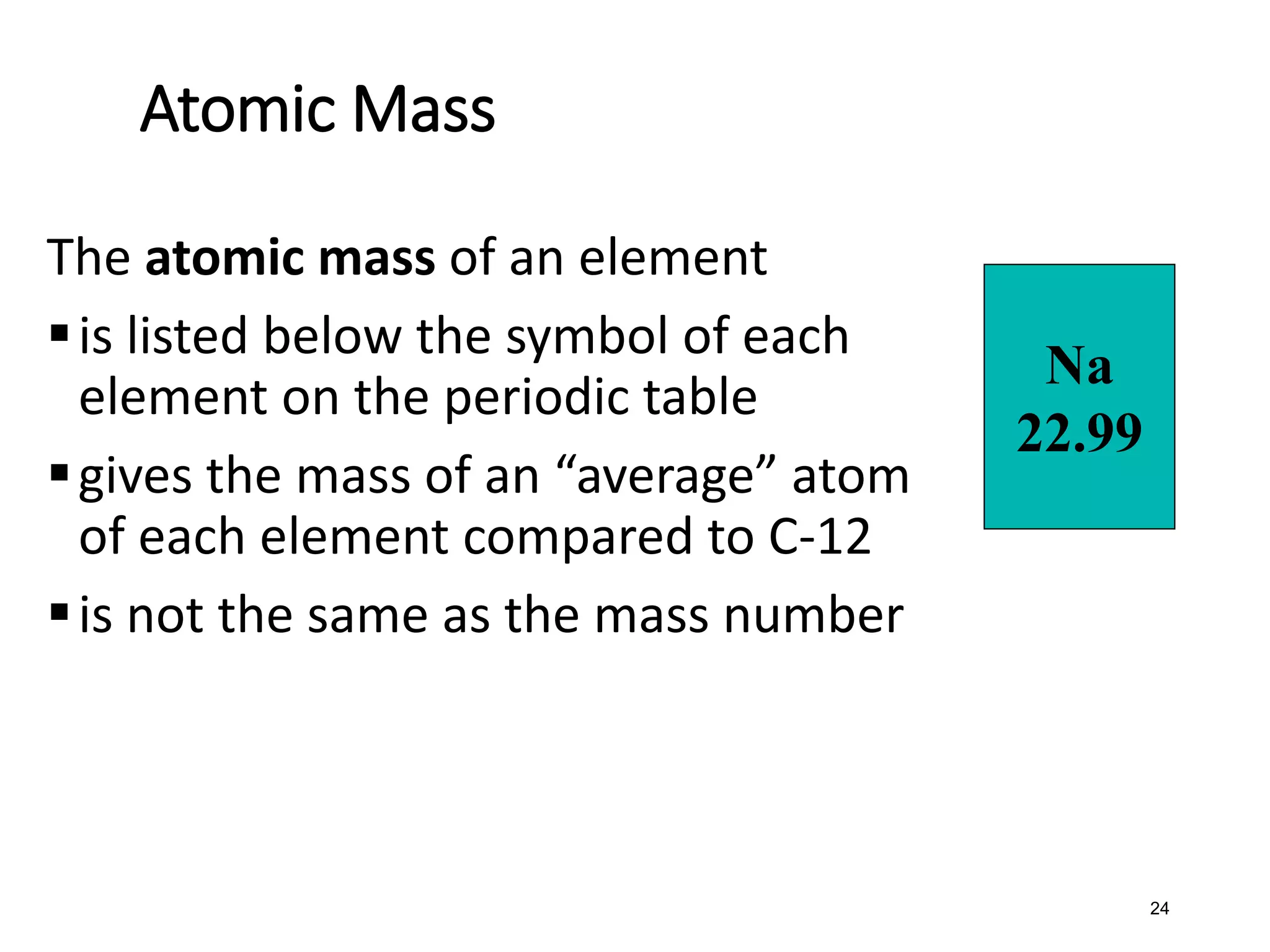
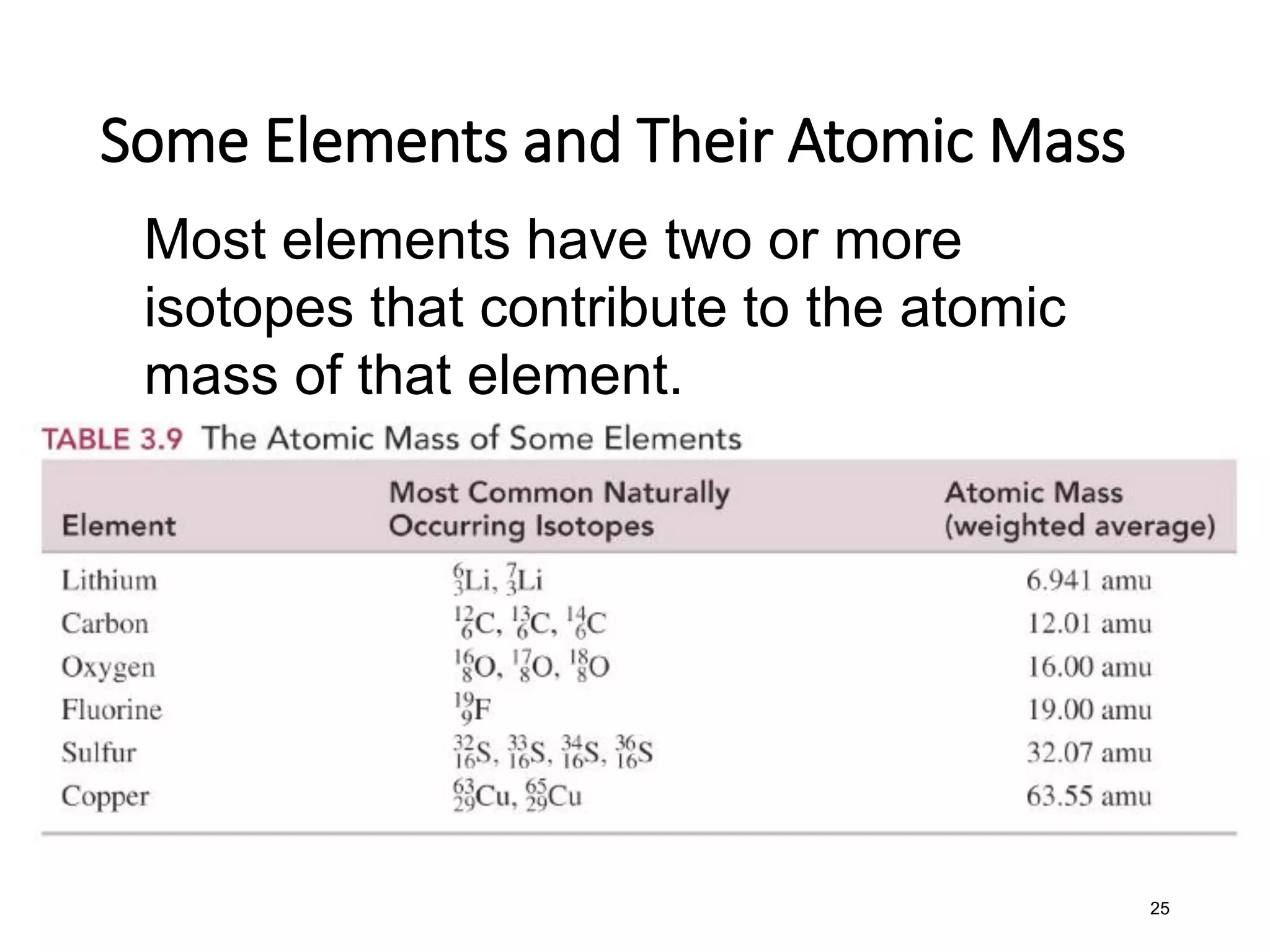
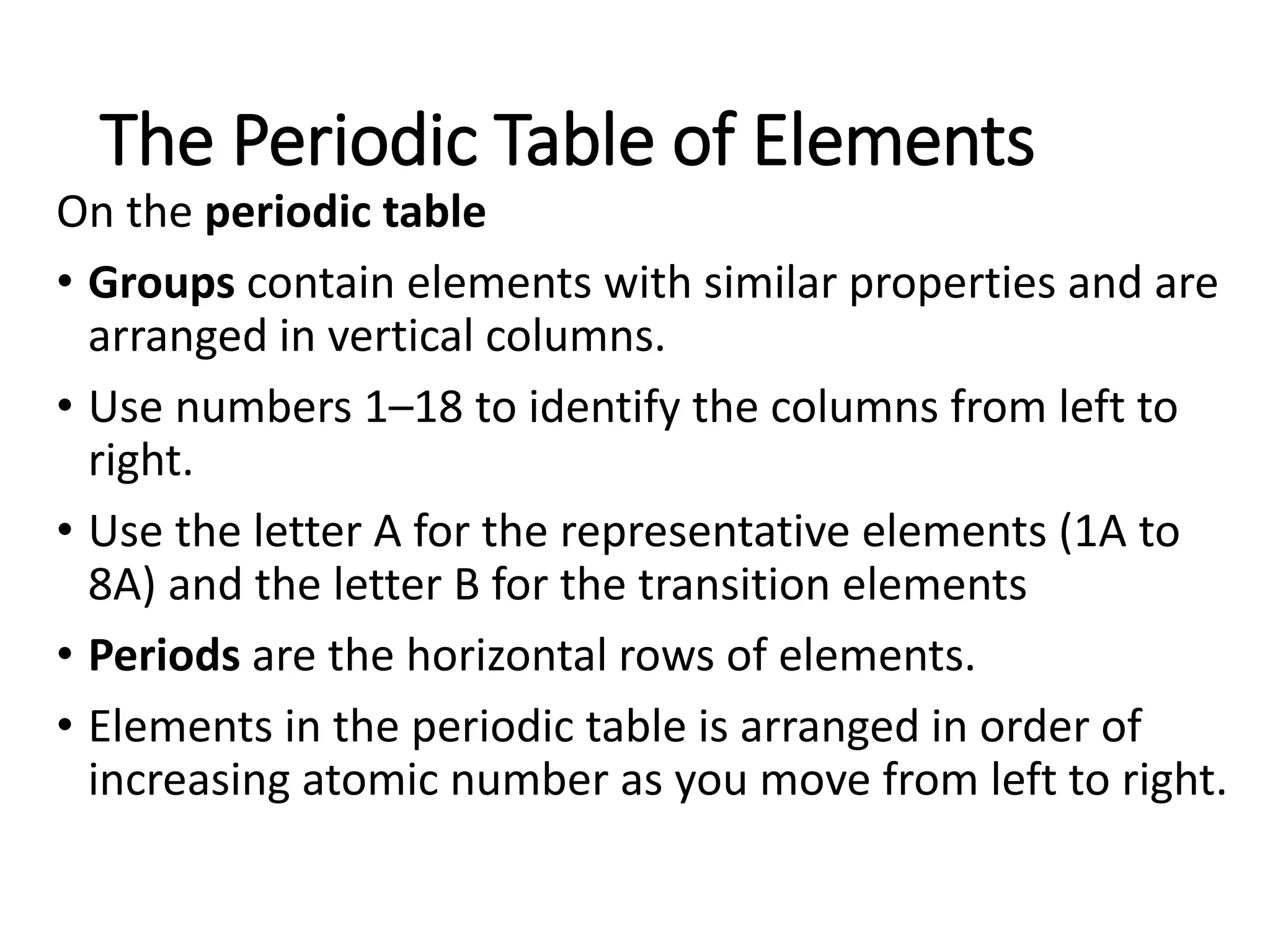
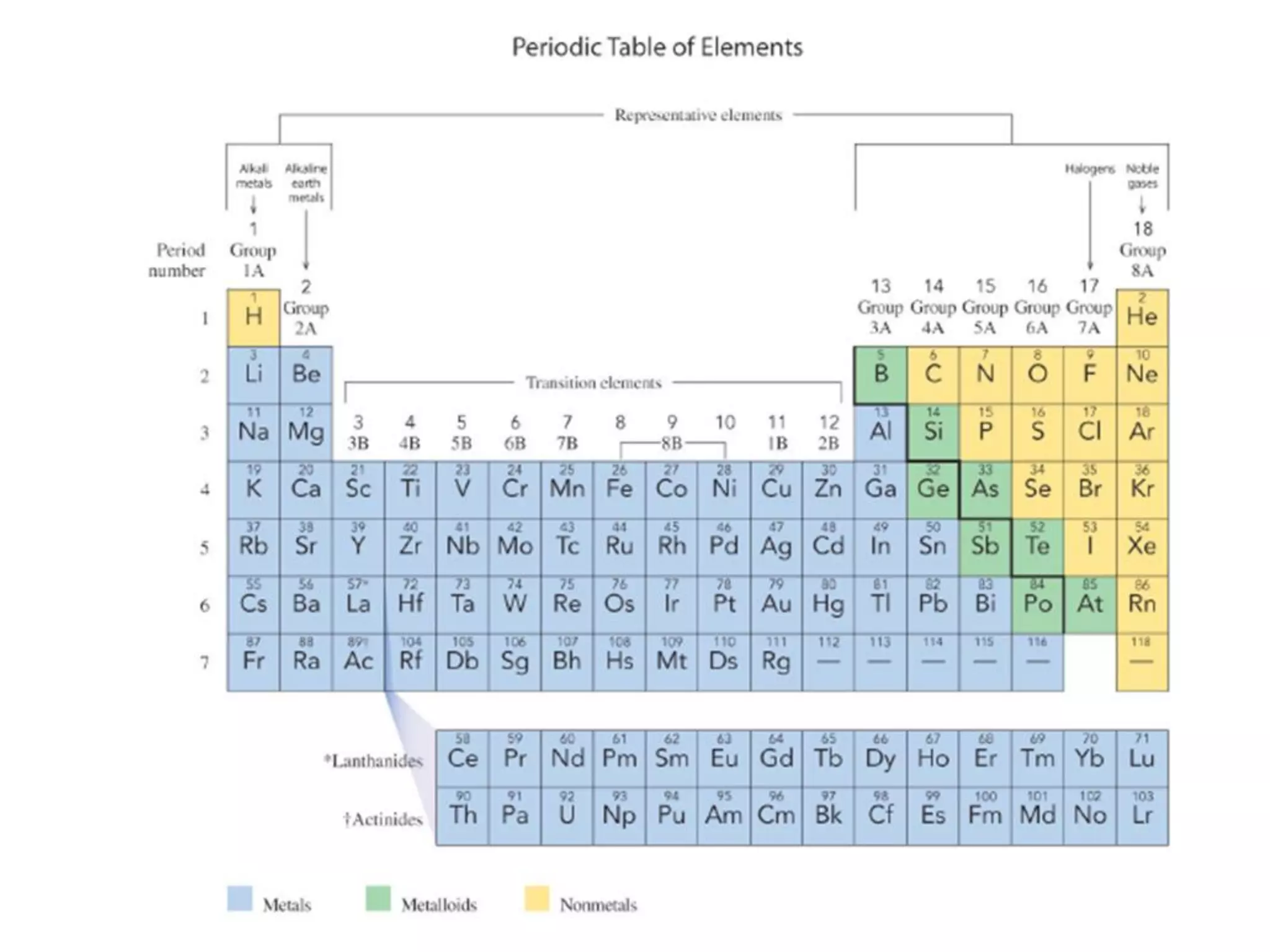
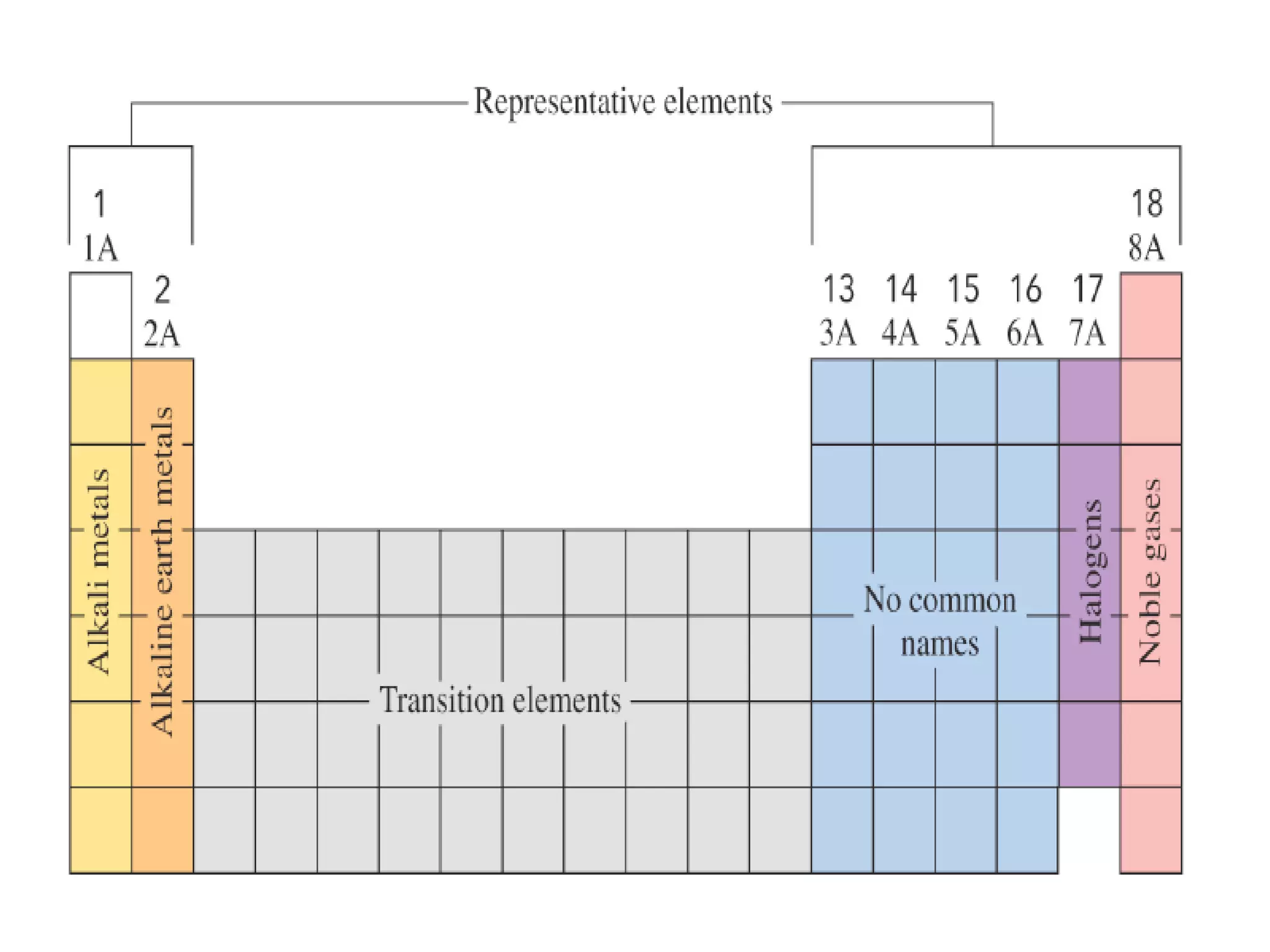
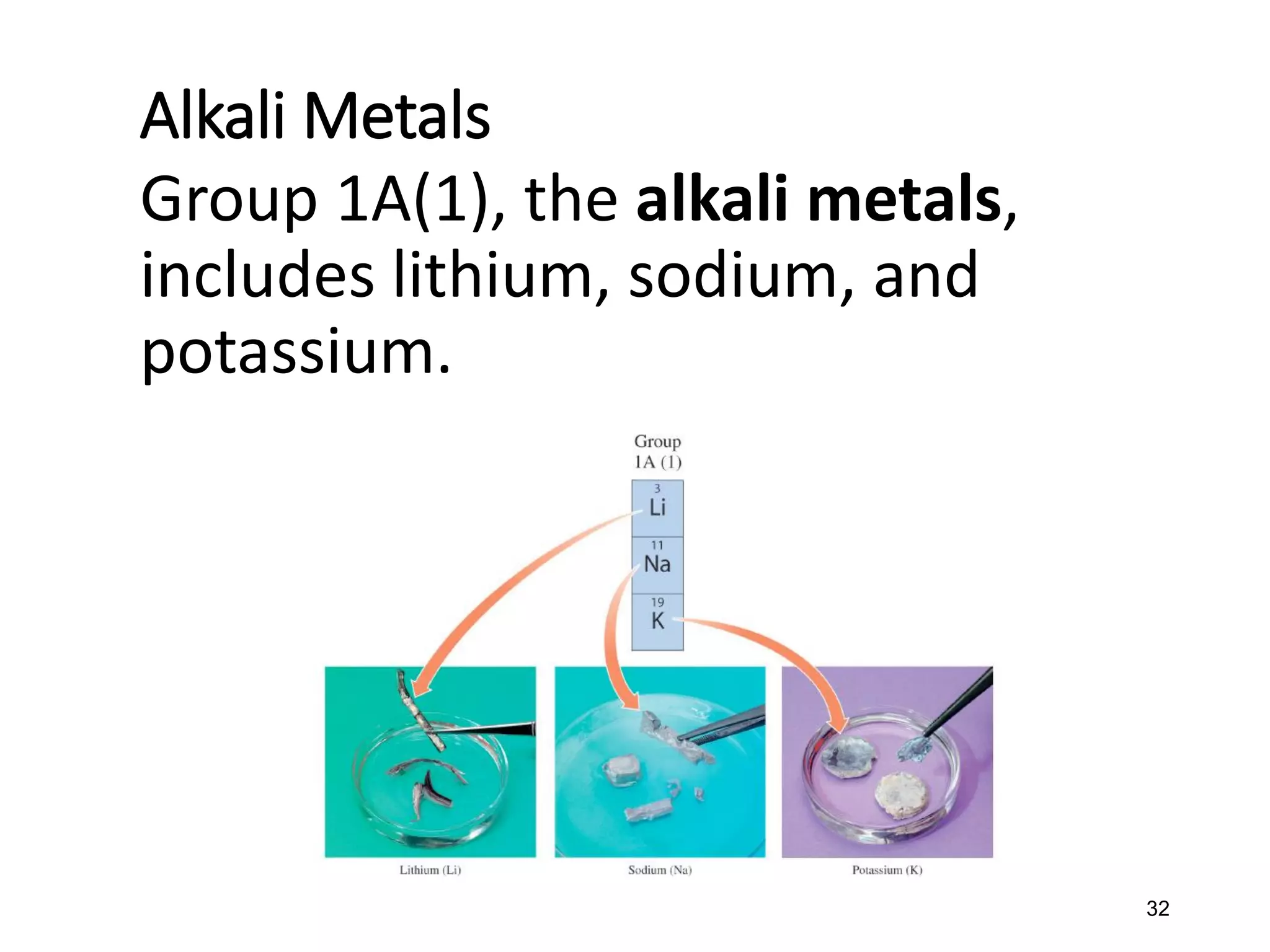
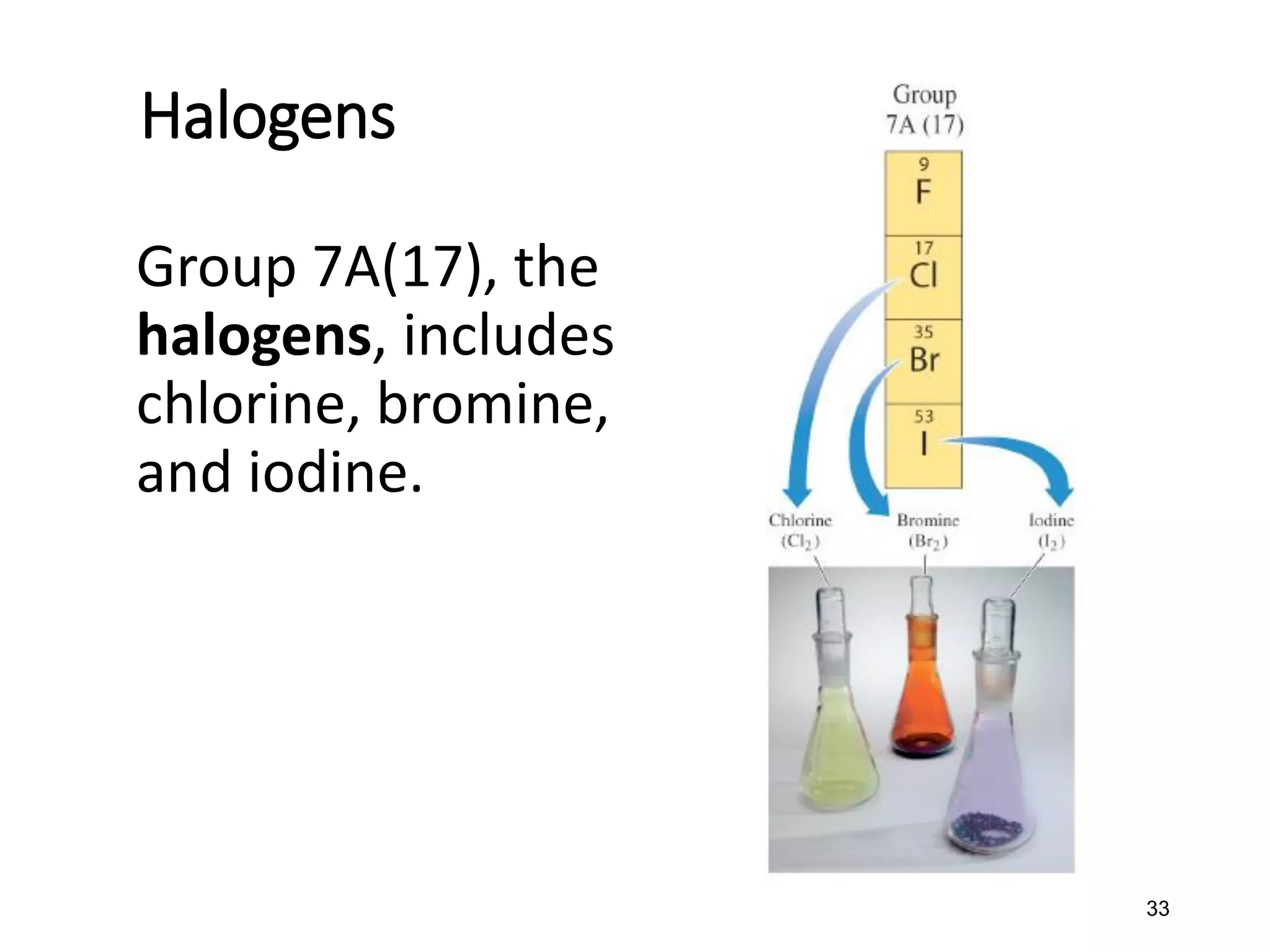
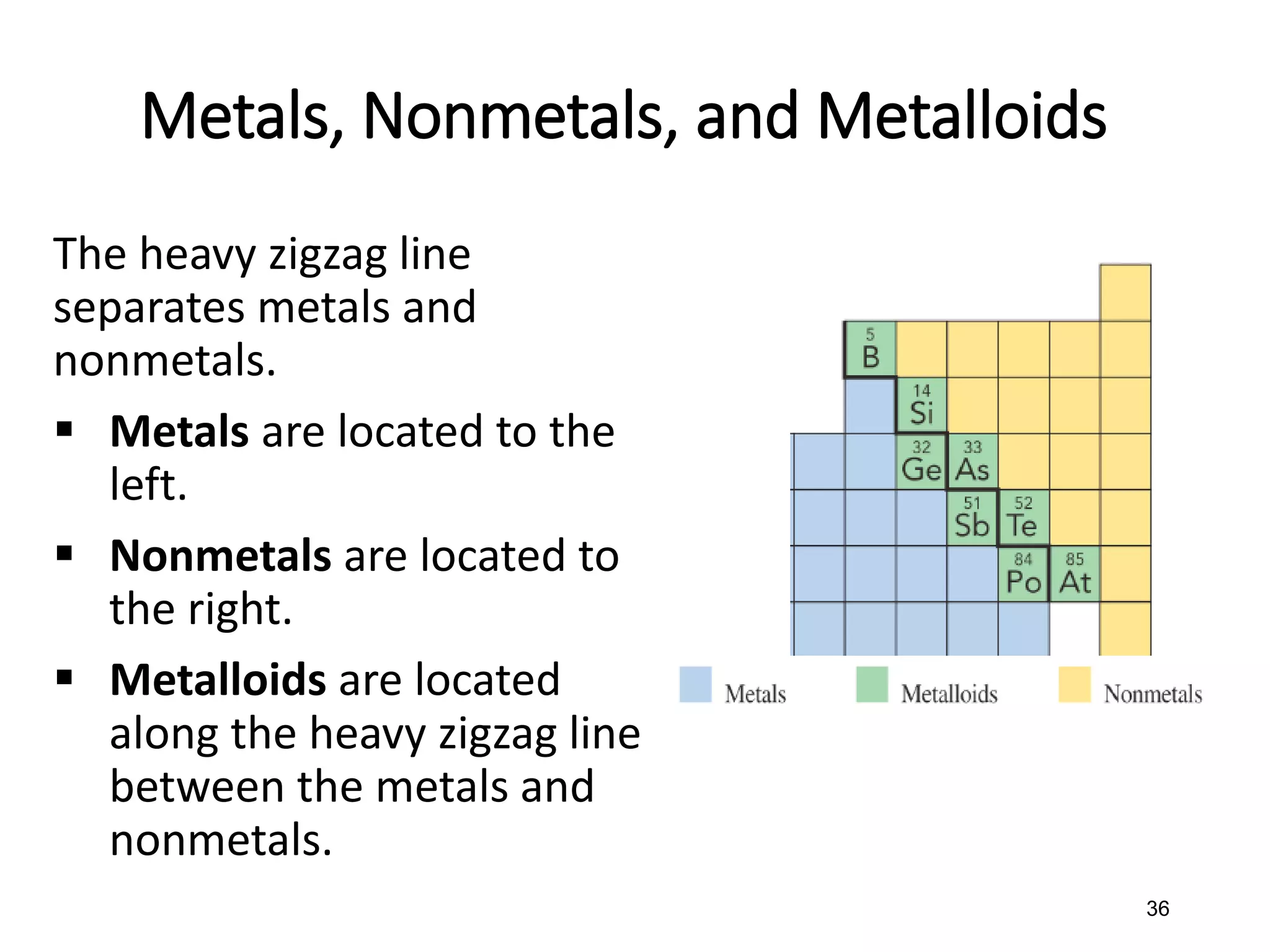
The document discusses the components and structure of atoms. It defines elements, molecules, compounds, and mixtures. Atoms consist of subatomic particles including protons, neutrons, and electrons. The number of protons determines the element, while the number of neutrons distinguishes between isotopes of the same element. The periodic table arranges elements based on atomic number and properties. Elements are classified as metals, nonmetals, and metalloids based on their properties. Valence electrons determine an element's chemical properties and reactivity. Atomic size and ionization energy depend on position in the periodic table.







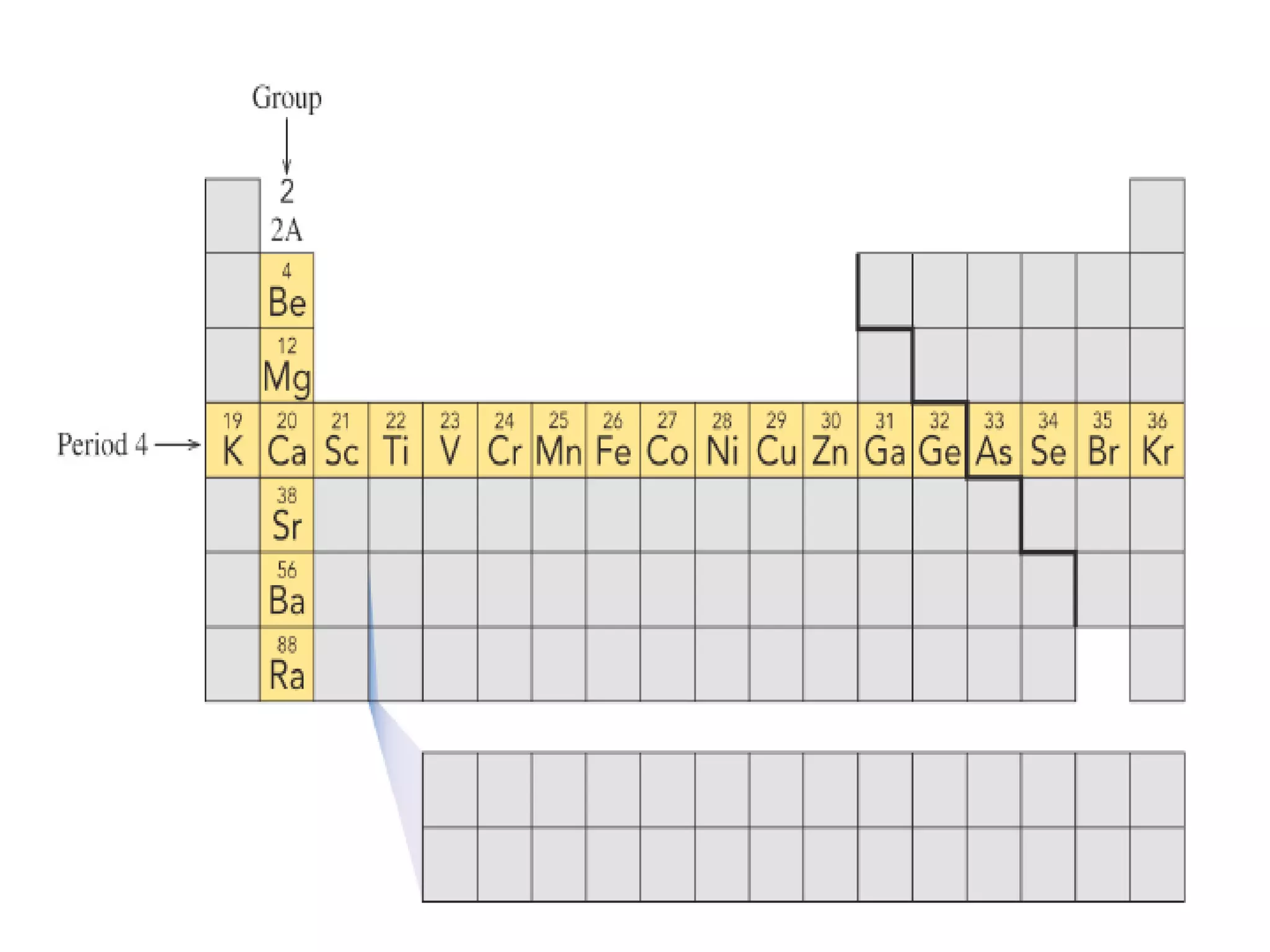



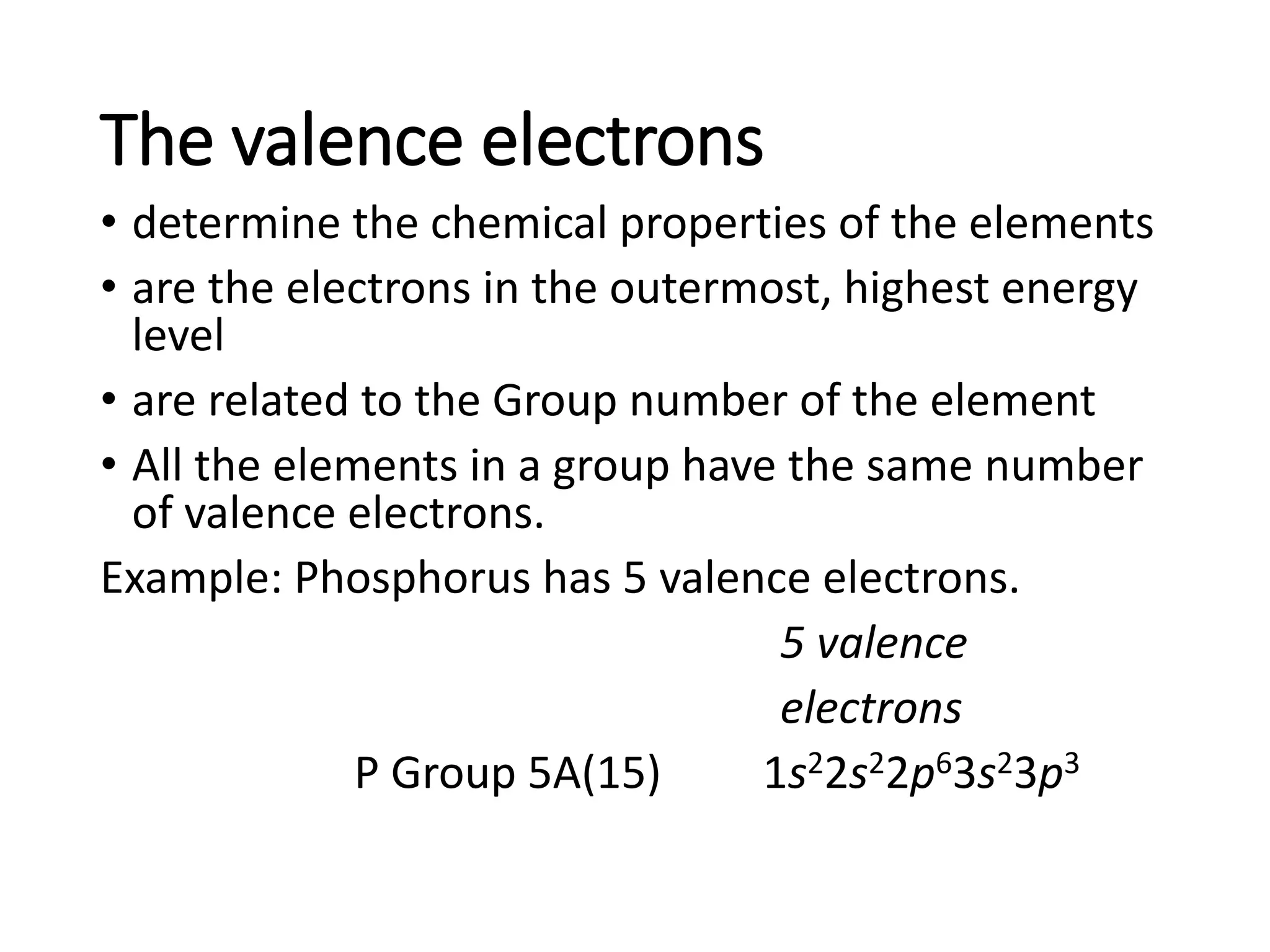
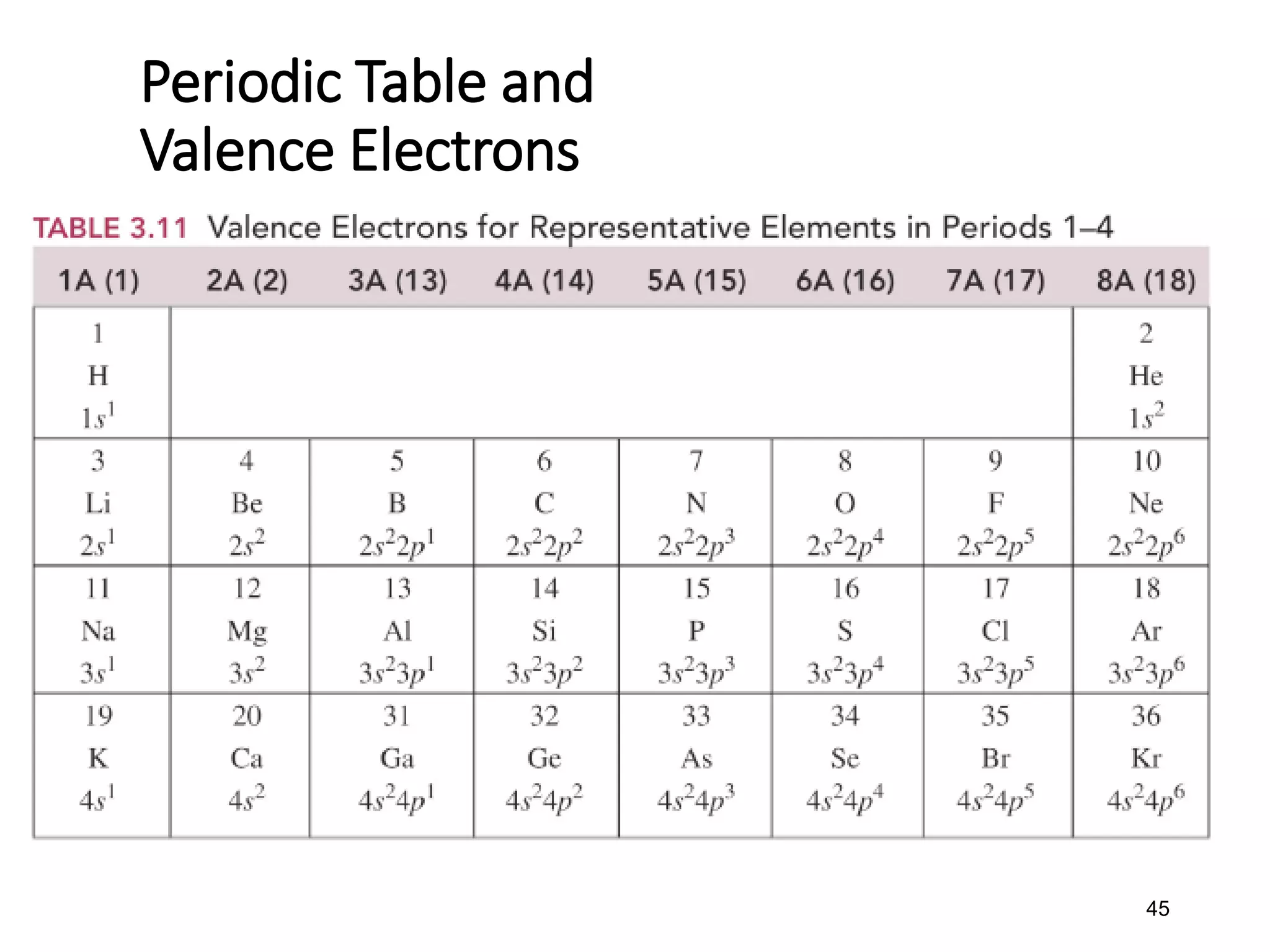
![•Example: Elements in Group
2A(2) have two (2) valence
electrons.
• Be 1s22s2
• Mg 1s22s22p63s2
• Ca [Ar]4s2
• Sr [Kr]5s2](https://image.slidesharecdn.com/week2-d2-componentsofmatter-231004001337-972366bb/75/week2-d2-Components-of-Matter-pdf-44-2048.jpg)