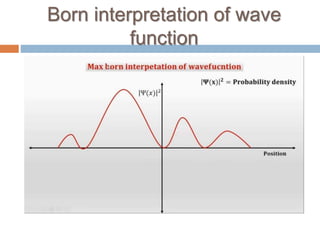
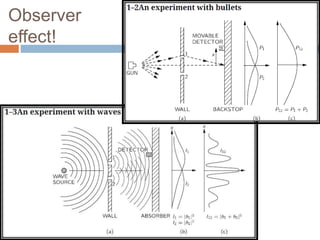
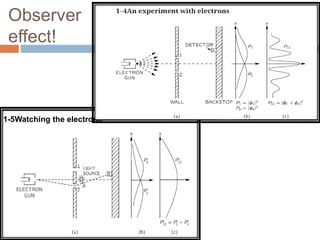
This document summarizes a seminar presentation on quantum physics. It introduces key concepts in quantum mechanics like wave functions, Schrodinger's equation, and the observer effect. Some quantum phenomena are explained briefly, like quantum superposition, tunneling, and spin. Applications of quantum physics are discussed, including quantum computing and technologies like lasers. Sources for further learning about quantum mechanics online are also provided.