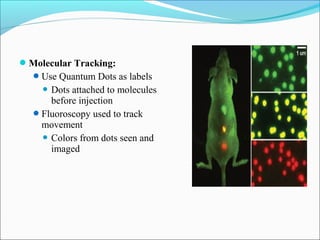
Ankit Nautiyal presented on the science and applications of quantum dots. Quantum dots are nanometer-sized semiconductor crystals that fluoresce at different wavelengths depending on their size. Their size-dependent properties make them useful for applications such as biological imaging, cancer detection, LED screens, solar cells, and molecular tracking. Specifically, quantum dots can be attached to molecules to track cell movement and monitor diseases like cancer over long periods of time. Their tunable light emission also allows for flexible, colorful LED and high-efficiency solar technologies. Further research continues to expand their beneficial uses in technology and medicine.