Embed presentation
Download to read offline


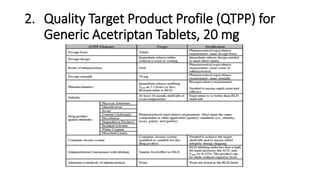
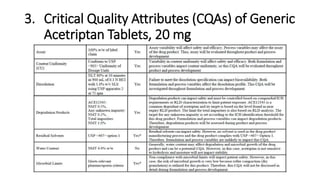
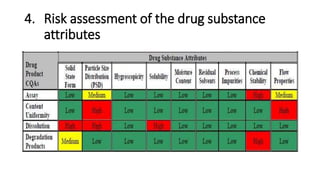
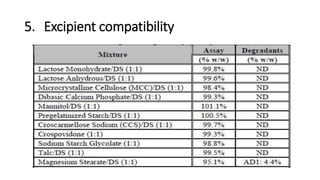
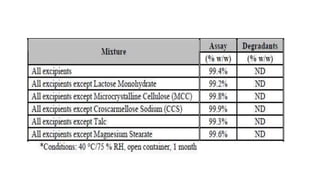
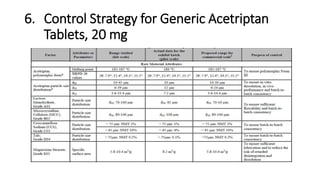
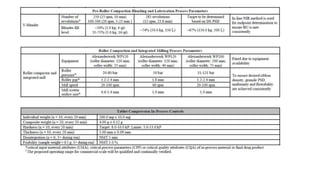


This document discusses applying Quality by Design (QbD) principles for developing a generic version of acetriptan tablets. It involves analyzing the reference listed drug to understand its clinical effects, pharmacokinetics, drug release properties, physicochemical characteristics and composition. This is used to establish a Quality Target Product Profile and identify the Critical Quality Attributes for the generic acetriptan tablets. A risk assessment of the drug substance attributes and excipient compatibility studies are also conducted. Finally, a control strategy is developed for the generic acetriptan tablets to ensure they meet the target product quality standards.









