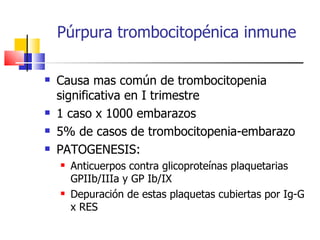
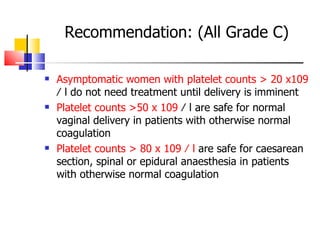
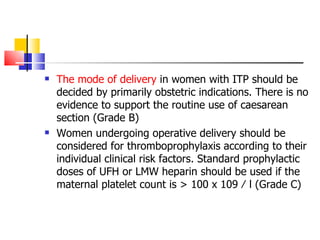
The document discusses thrombocytopenia associated with pregnancy. It notes that thrombocytopenia commonly occurs in the third trimester of pregnancy and affects around 7% of pregnancies. Thrombocytopenia can be isolated, immune-mediated (idiopathic thrombocytopenic purpura), drug-induced, or associated with other systemic disorders like preeclampsia. Diagnosis involves blood tests and ruling out other potential causes. Treatment depends on platelet count and severity but may involve corticosteroids, IVIG, or splenectomy in severe cases. Risk of bleeding in infants is generally low but platelet counts should be monitored.


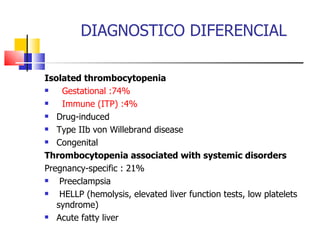
![DIAGNOSTICO DIFERENCIAL Not pregnancy specific : Thrombotic microangiopathies: Thrombotic thrombocytopenic purpura (PTT) Hemolytic uremic syndrome (SUH) Systemic lupus erythematosus (LES) Antiphospholipid antibodies (SAF) Disseminated intravascular coagulation (CID) Viral infection (human immunodeficiency virus [HIV],Epstein-Barr virus [EBV], cytomegalovirus [CMV]) Bone marrow dysfunction Nutritional deficiency Hypersplenism](https://image.slidesharecdn.com/ptipreg-1233424255573623-1/85/PTI-4-320.jpg)