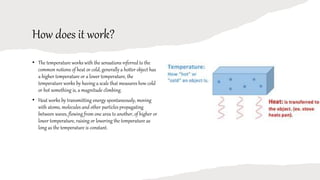
Heat is a form of energy that flows from hotter objects to cooler objects and can be produced through bending, chemical reactions, or collisions. Temperature is a measure of how hot or cold an object is on a given scale. Heat travels through conduction, convection, or radiation. While heat is the transfer of thermal energy, temperature is a property relating to average molecular kinetic energy. Materials that allow rapid heat transfer are conductors, while insulators do not allow easy heat passage. Heat is found in everyday life through cooking, warming, engines, and generating energy.