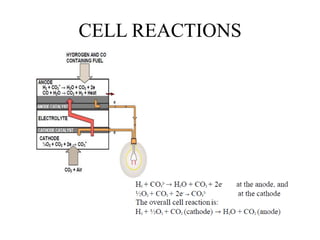
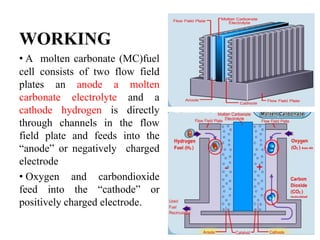
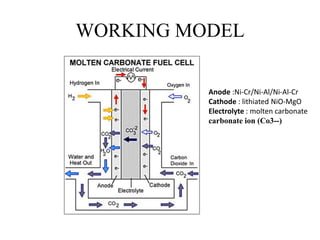
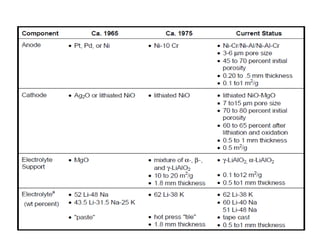
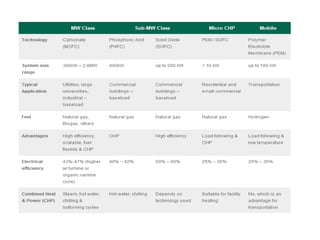
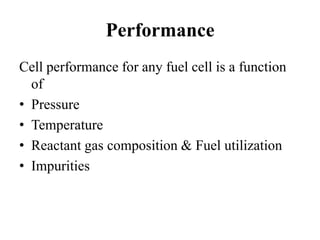
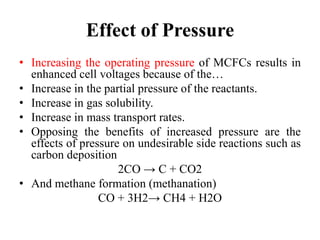
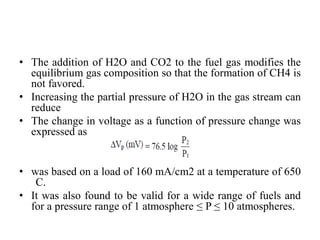
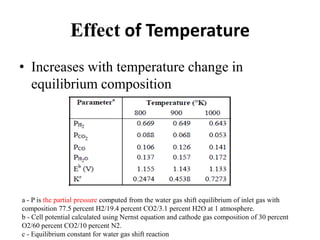
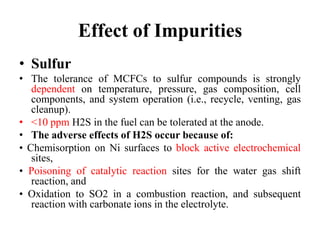
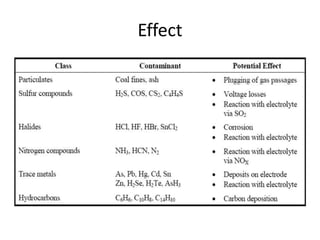
The document summarizes the workings and performance of molten carbonate fuel cells. It discusses that molten carbonate fuel cells operate at around 650°C, allowing the use of non-noble metal catalysts. The key reactions and components of the fuel cell are described, including the hydrogen oxidation reaction at the anode and oxygen reduction reaction at the cathode. Performance is affected by various factors like pressure, temperature, reactant gas composition and utilization, and impurities. Advantages include high efficiency and tolerance for internal reforming, while disadvantages include intolerance to sulfur and liquid electrolyte handling challenges.