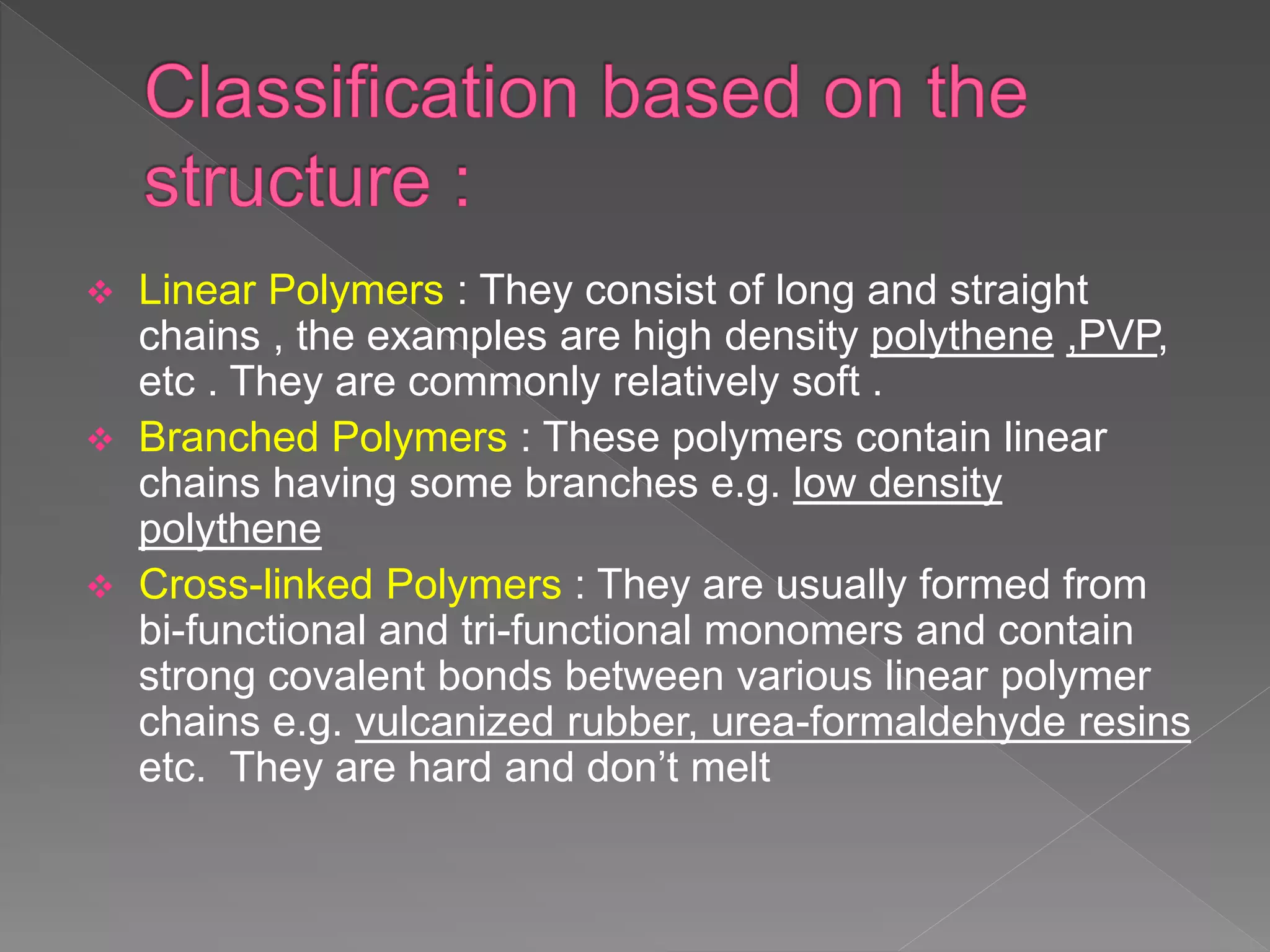
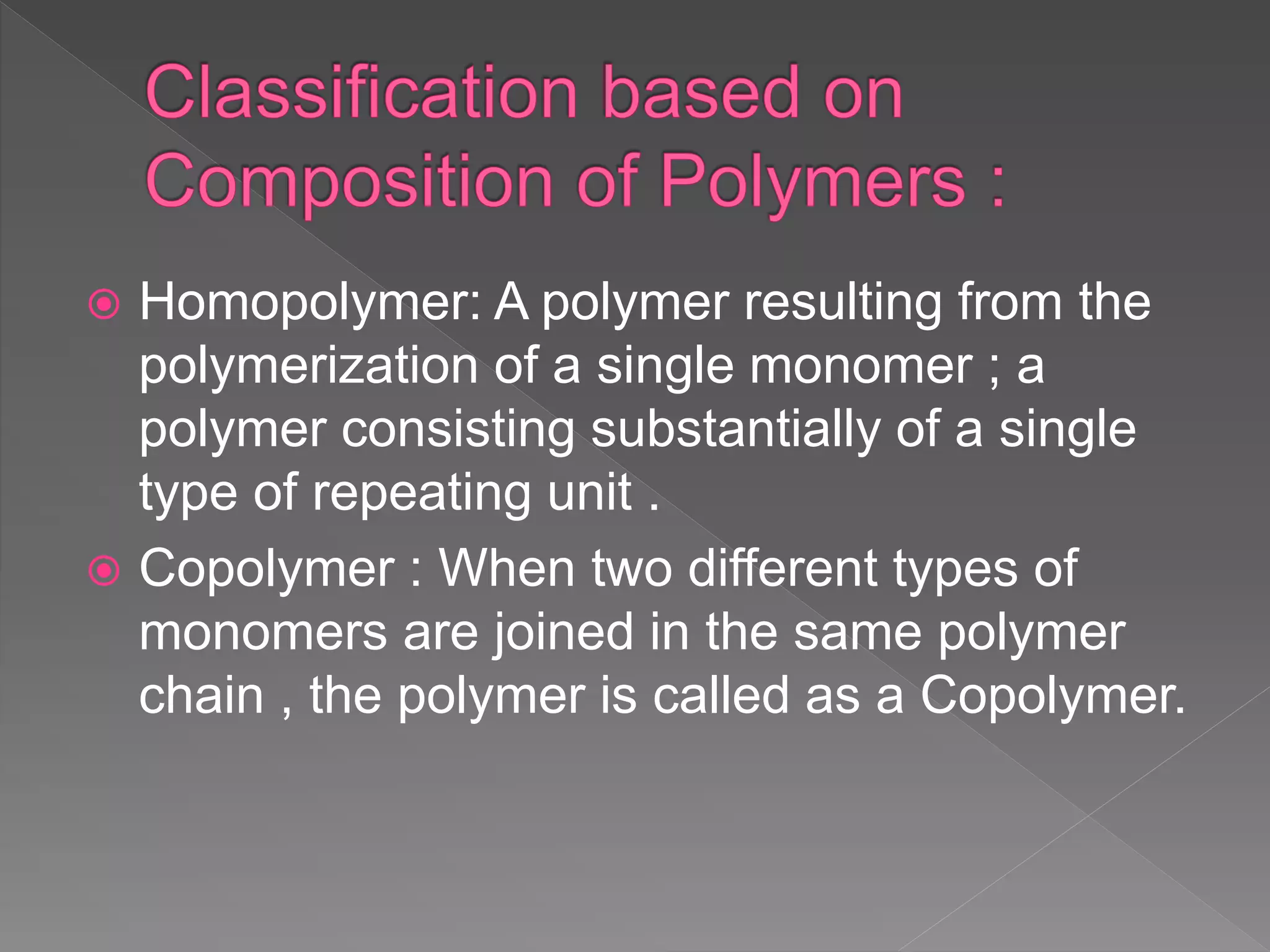
This document discusses polymers including their classification, types, characteristics, and applications. Polymers are large molecules composed of many repeating structural units. They can be natural, semi-synthetic, or synthetic and are classified based on source, structure, composition, polymerization method, molecular forces, and backbone chain. Common types include linear, branched, and cross-linked polymers as well as homopolymers and copolymers. Polymerization occurs through addition or condensation reactions. Polymers exhibit properties like being thermoplastic, thermosetting, or elastic. They have various applications in medicine, consumer goods, industry, and sports.