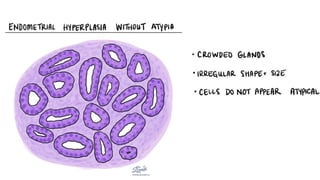
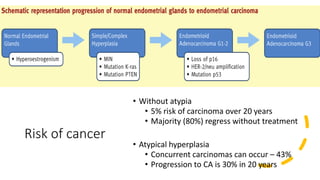
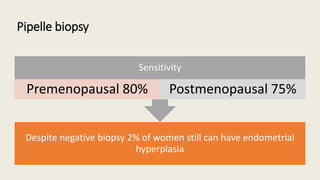
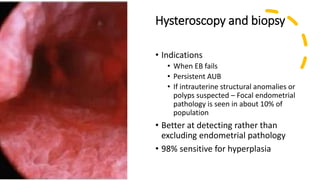
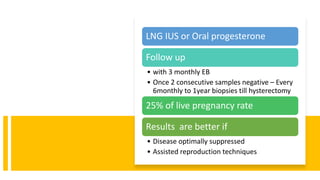
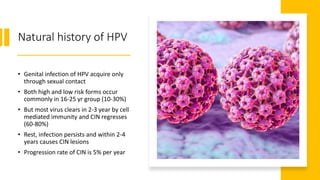
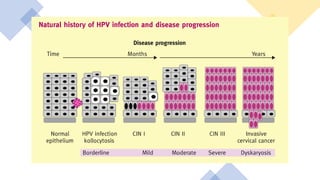
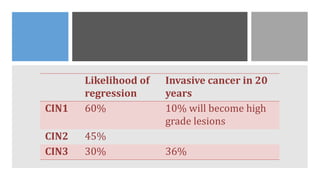
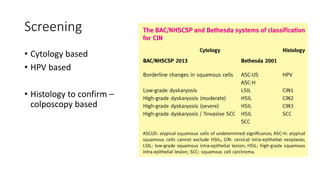
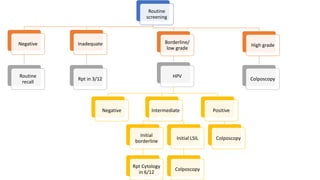
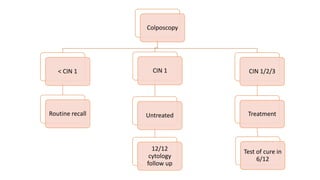
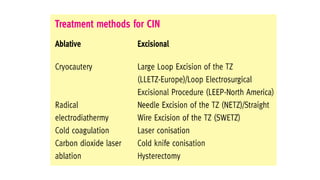
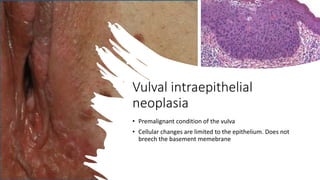
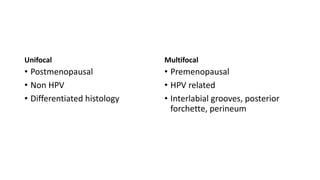
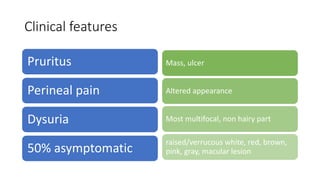
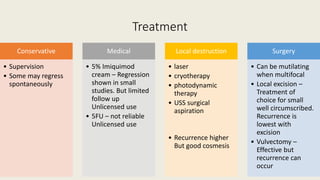
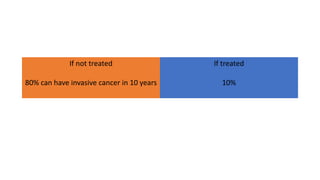
This document discusses several pre-malignant gynaecological conditions including endometrial hyperplasia, cervical pre-invasive disease, and vulval intraepithelial neoplasia. It covers the epidemiology, risk factors, clinical presentation, diagnosis, classification, and treatment options for each condition. Endometrial hyperplasia can progress to endometrial cancer if left untreated, and treatment options include progesterone, hysterectomy, or endometrial ablation depending on severity. Cervical pre-invasive lesions are usually caused by HPV infection and may regress on their own, but higher grades have a risk of progressing to invasive cervical cancer without treatment. Screening and treatment modalities aim to identify and treat higher