Psammophilus dorsalis agamas were studied across urban and rural areas in India to understand how they persist in urban environments. The study found:
1) The agamas' diet in both areas was mainly composed of ants, indicating flexibility.
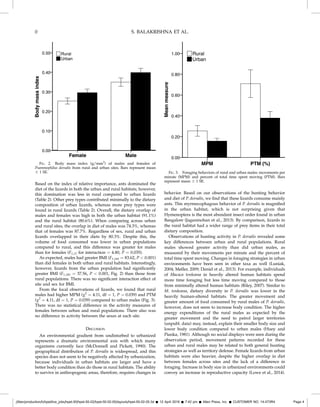
2) Dietary overlap was high between urban and rural populations (80.3%), and between males and females in each area (80-91%).
3) Surprisingly, urban agamas had higher body mass indices than rural ones, despite consuming a greater diversity and volume of prey in rural areas.
4) Males moved more in rural areas, likely due to higher energy expenditure, but all agamas managed to hunt enough in fragmented urban habitats.