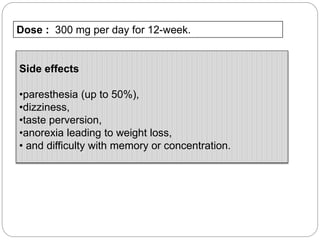
This document provides an overview of alcohol dependence, including its history, pharmacology, clinical criteria, mechanisms of dependence, and pharmacotherapy options. It discusses how alcohol is metabolized and absorbed in the body, its acute and chronic effects, and the neuropharmacological basis of dependence and withdrawal. Several pharmacotherapies for alcohol dependence are described in detail, including disulfiram, naltrexone, acamprosate, topiramate, ondansetron, gabapentin, pregabalin, SSRIs, and aripiprazole. The document also reviews genetic variations affecting alcohol metabolism and newer treatment targets beyond the three FDA-approved medications.