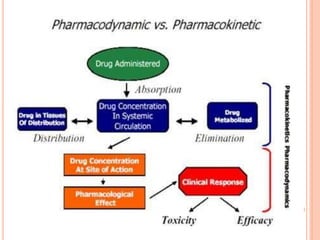
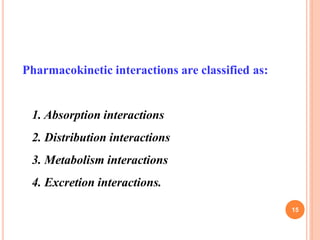
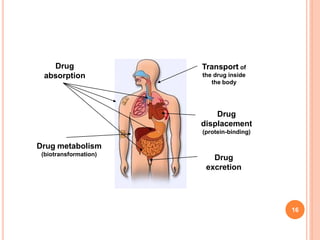
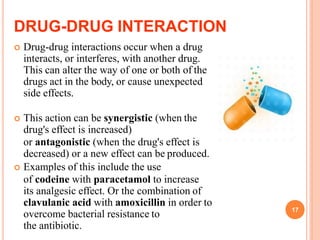
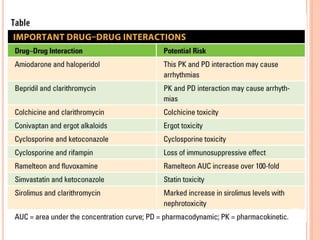
This document defines and classifies incompatibilities in pharmaceutical preparations. It discusses physical incompatibilities caused by insolubility, immiscibility, or liquification of solids. Chemical incompatibilities involve oxidation, hydrolysis, or other chemical reactions. Therapeutic incompatibilities are unintentional pharmacodynamic or pharmacokinetic drug interactions. The document also describes different types of drug interactions including pharmacodynamic, pharmacokinetic, drug-drug, drug-excipient, excipient-excipient, drug-food, and excipient-packaging interactions. It emphasizes the importance of informing doctors and pharmacists of all medications and supplements to prevent harmful drug interactions.