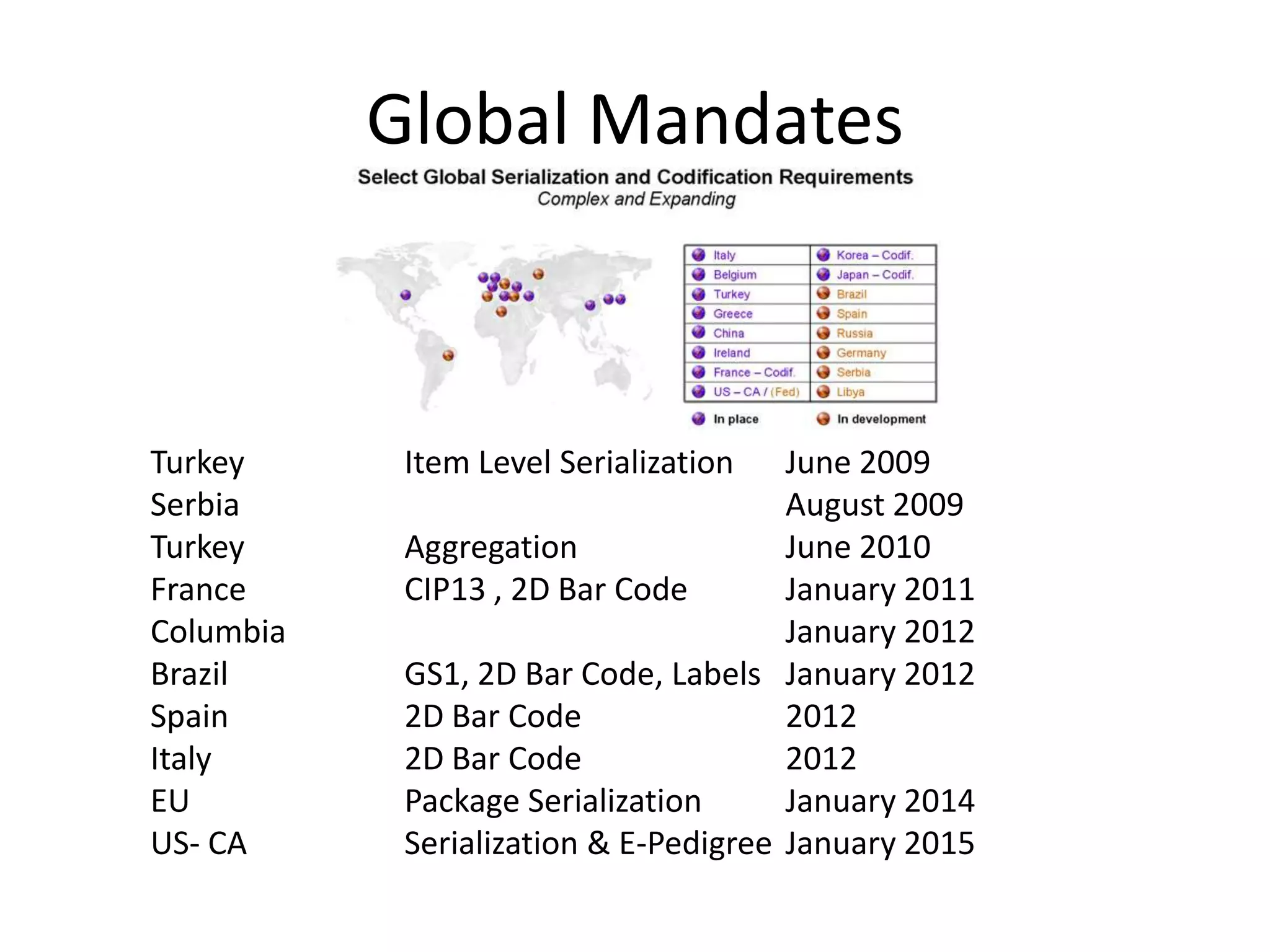
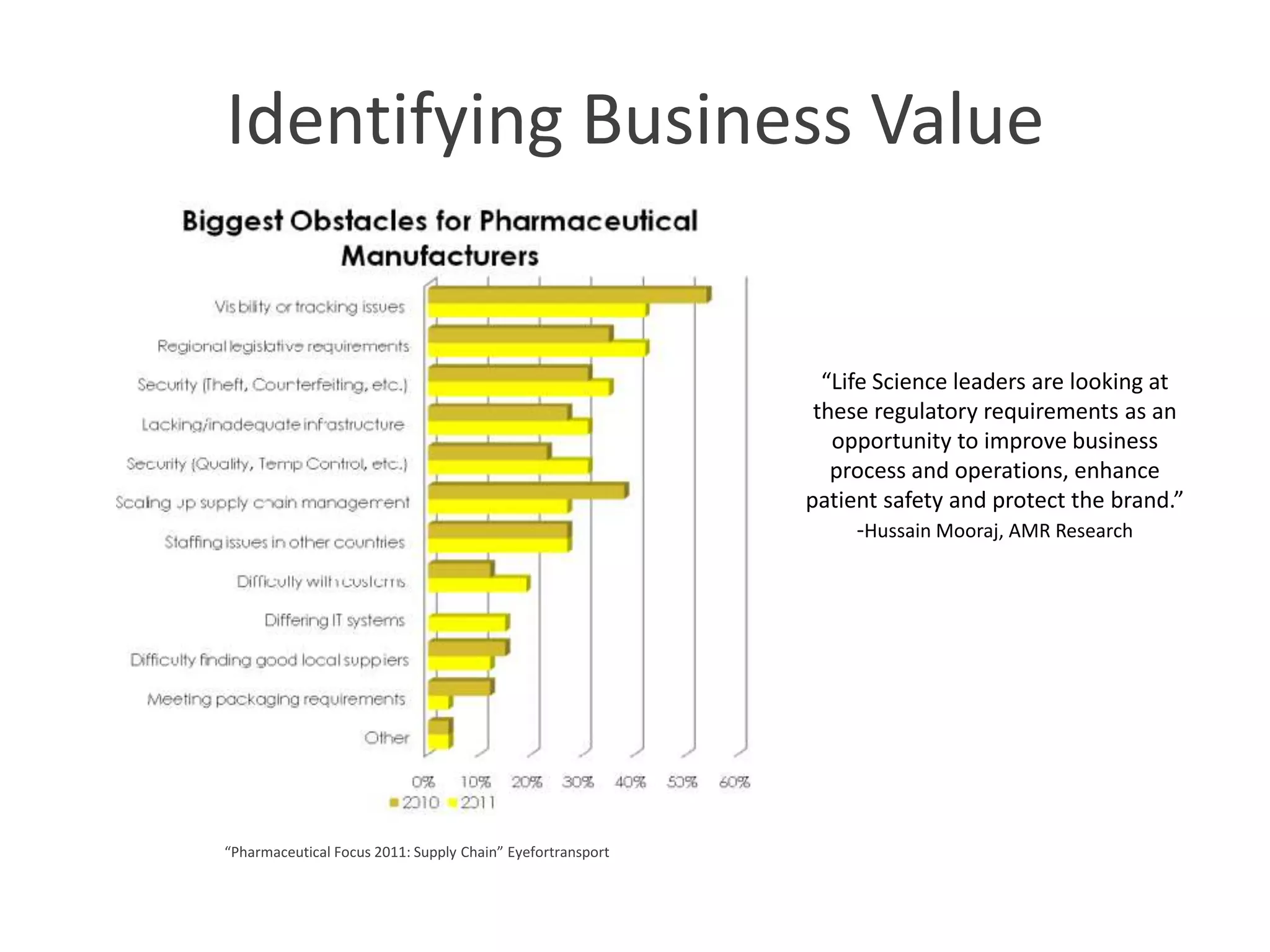
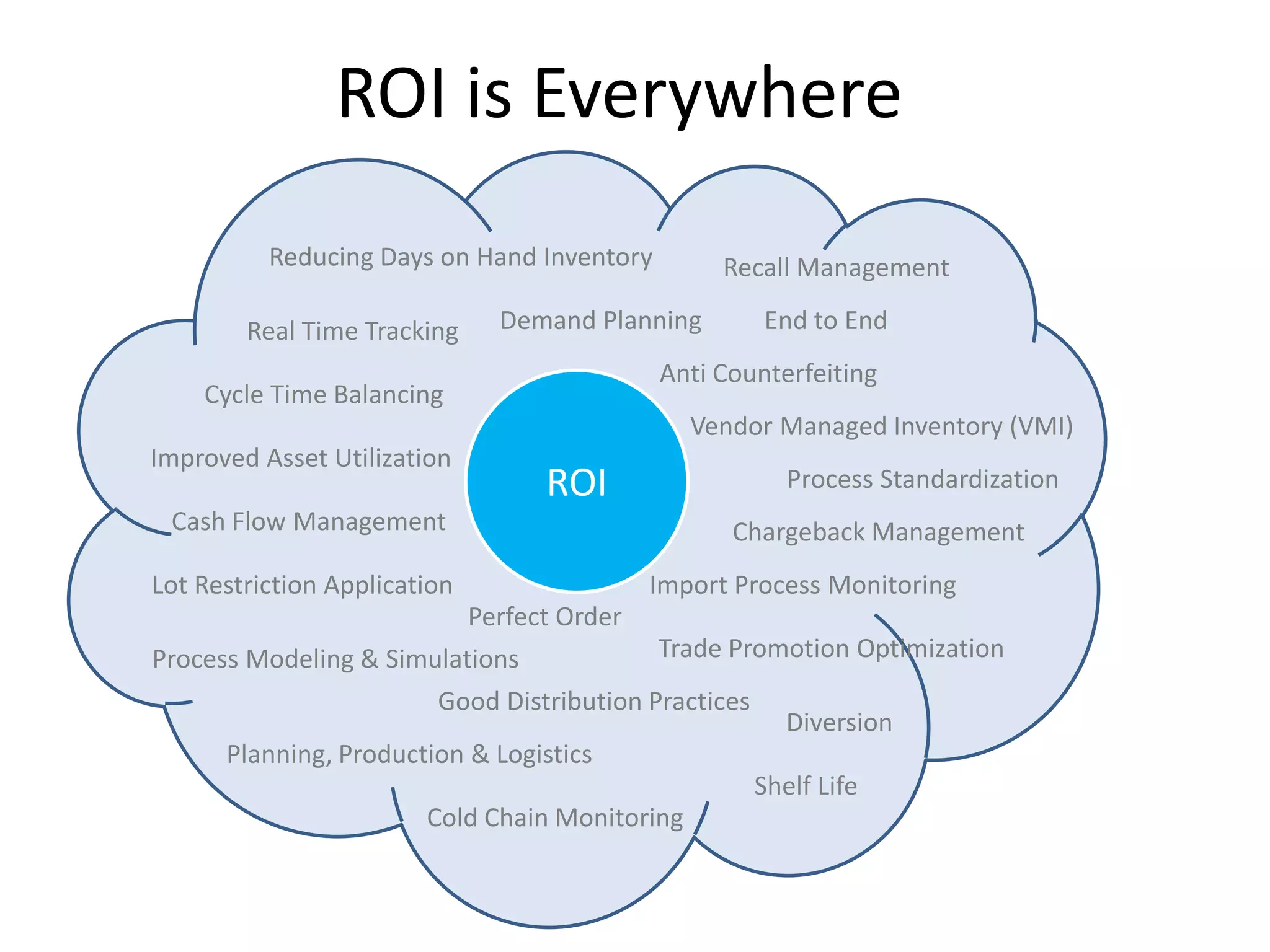
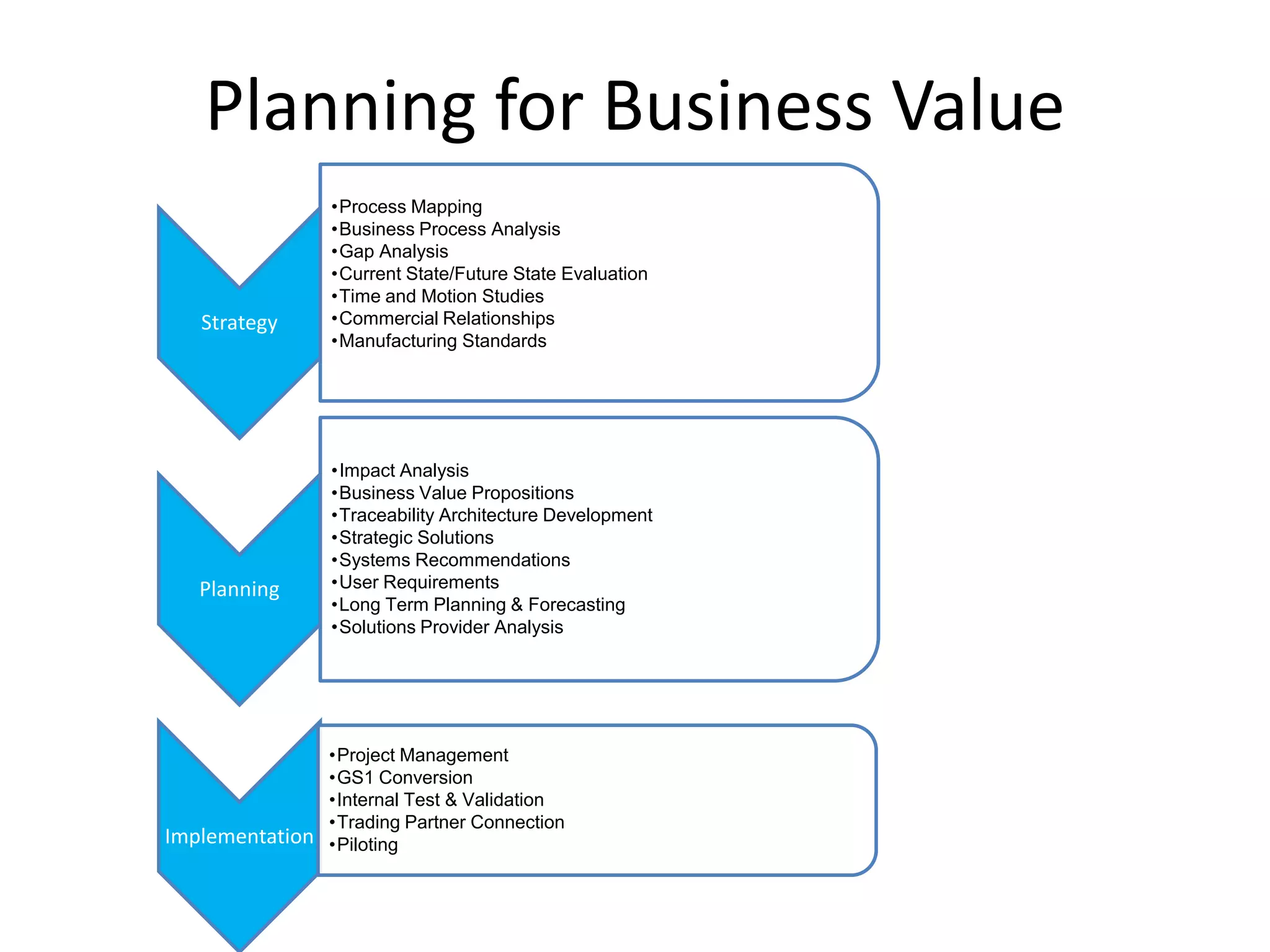
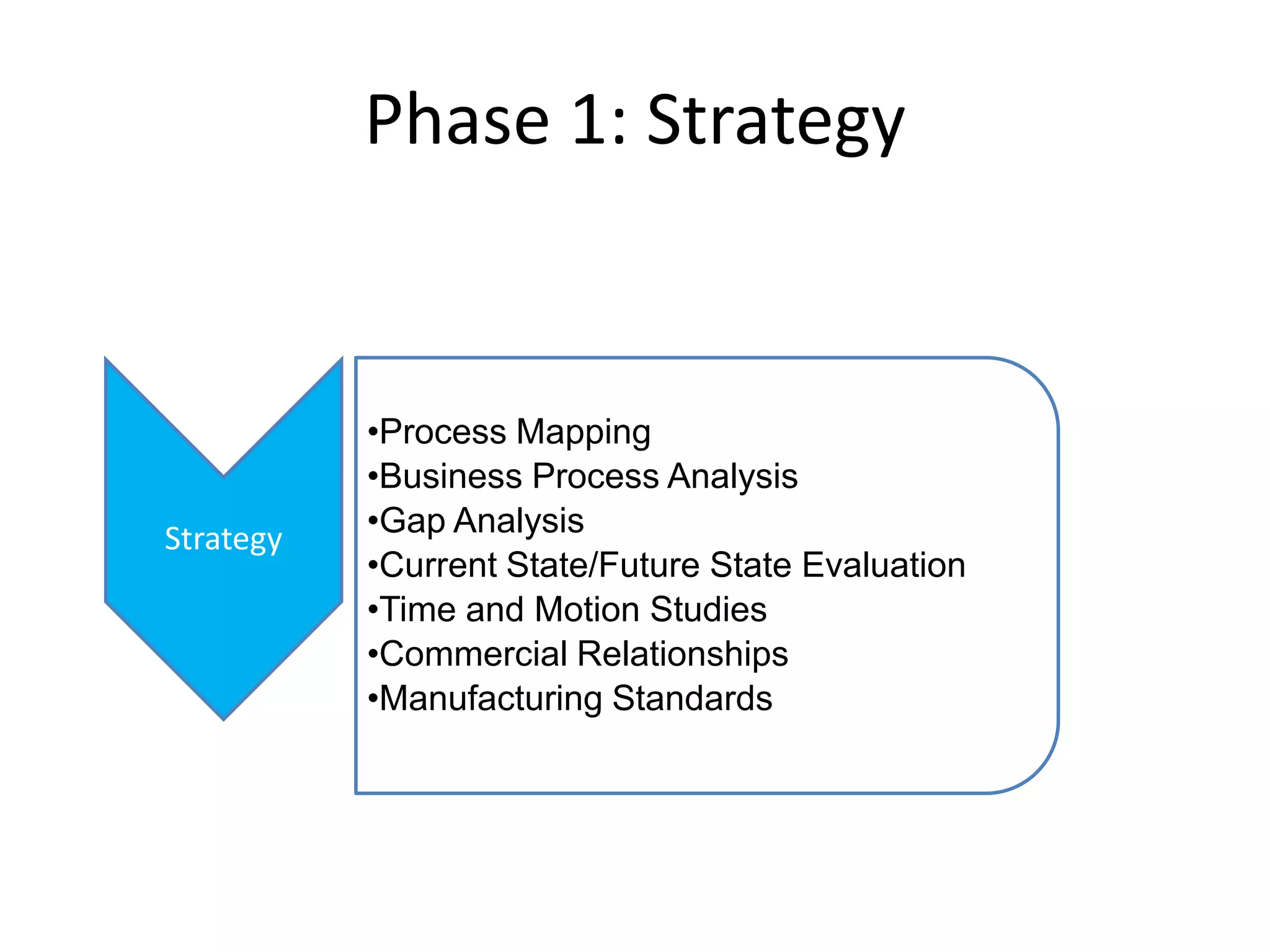
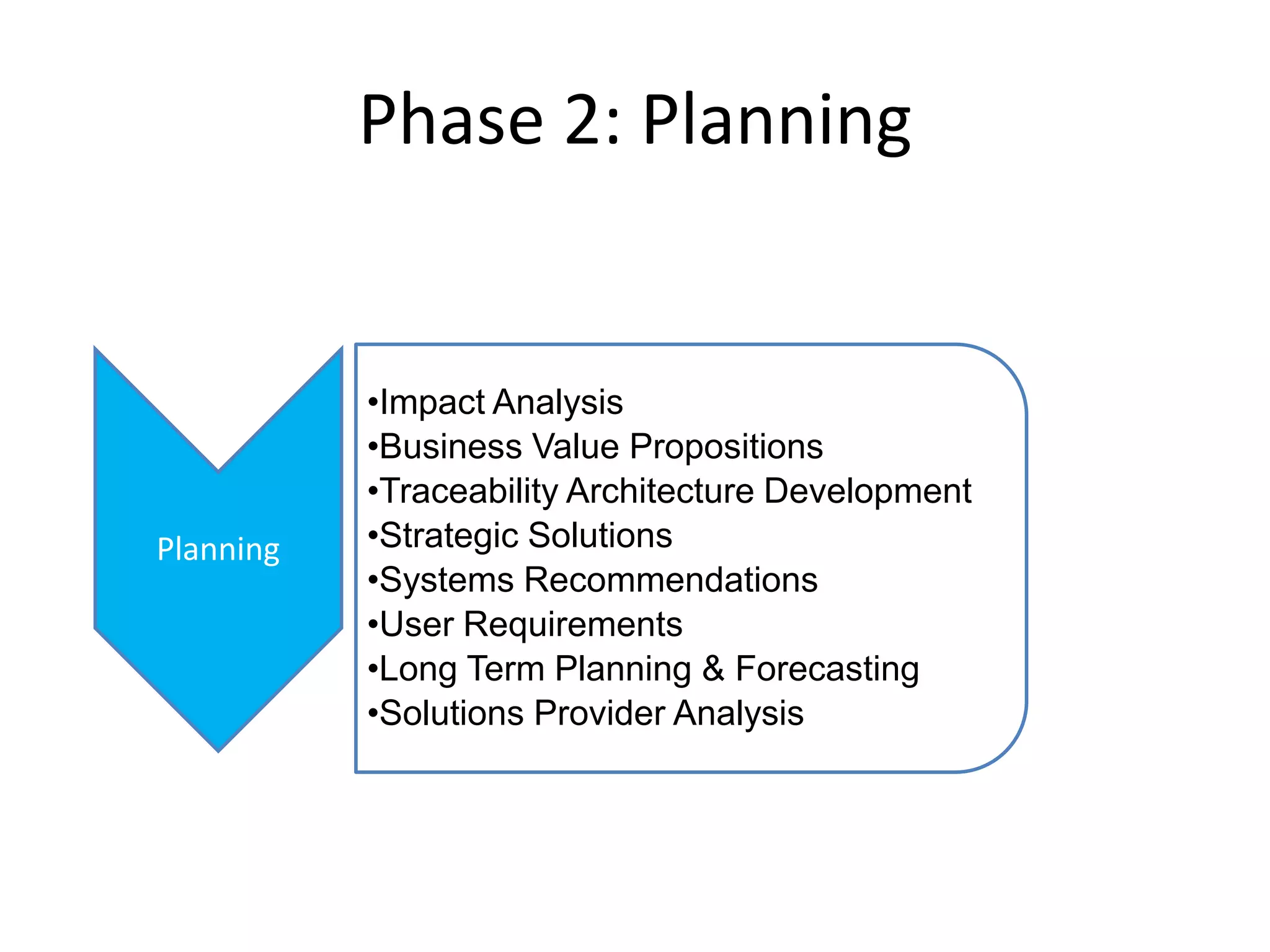
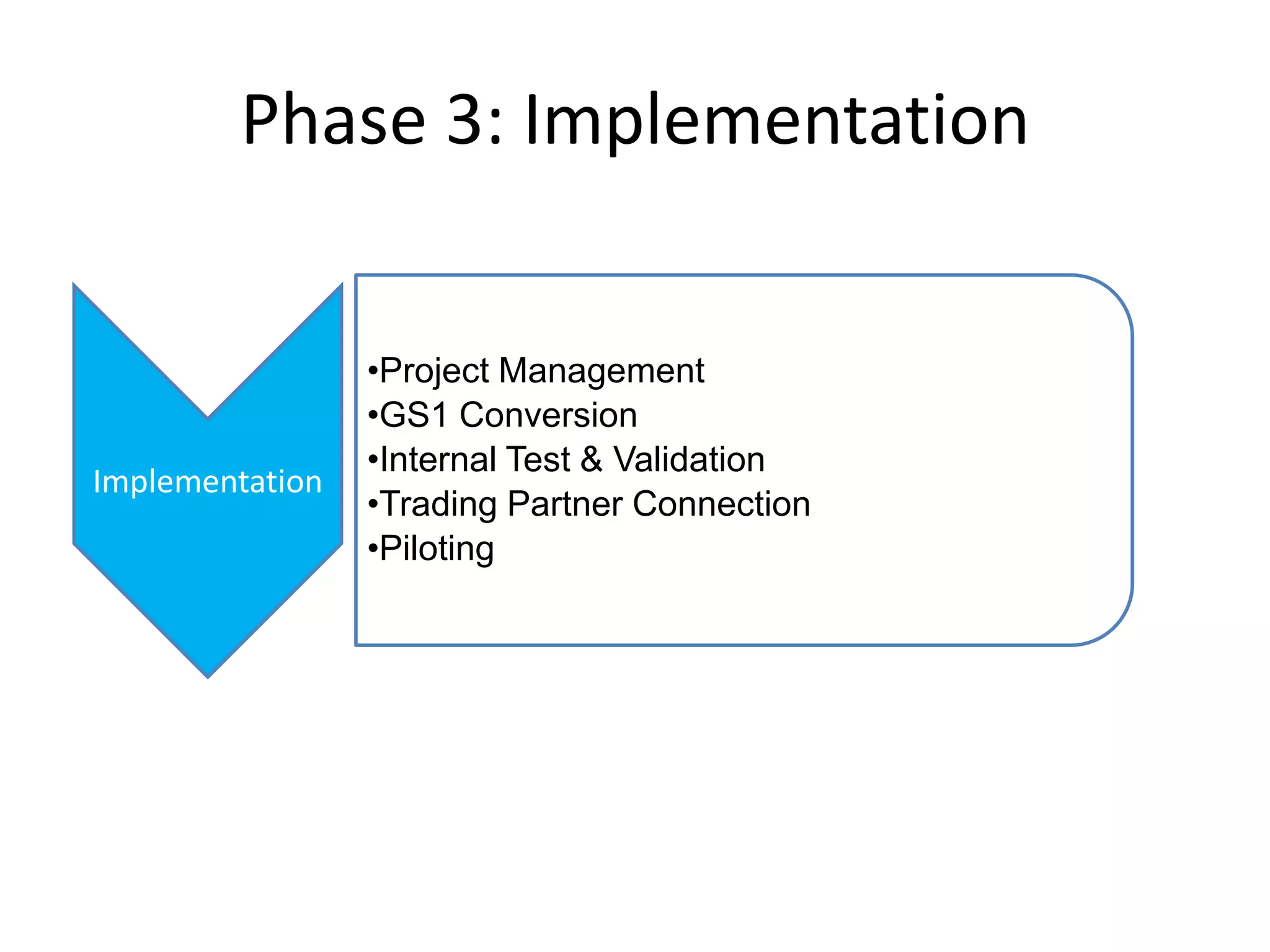
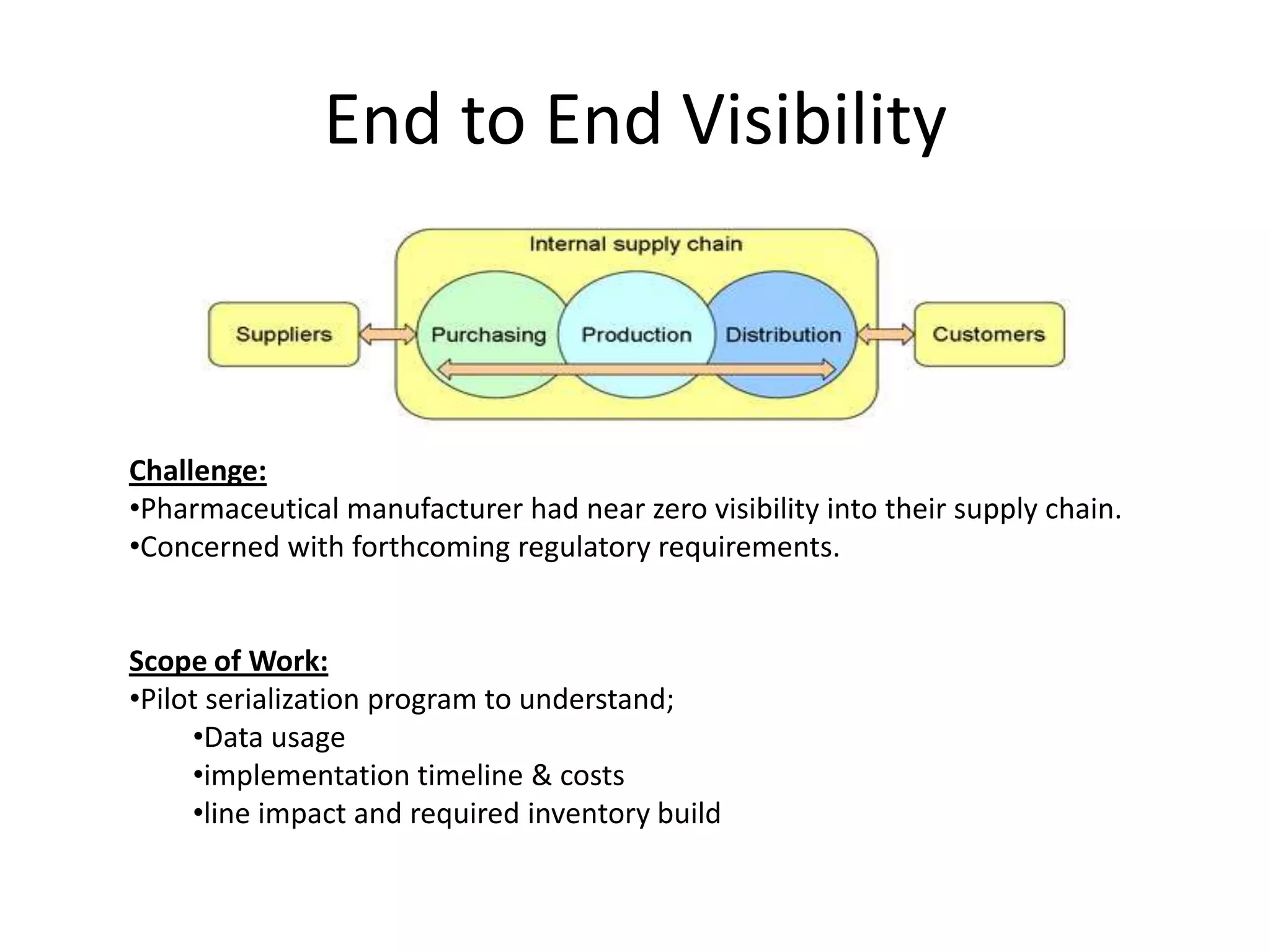
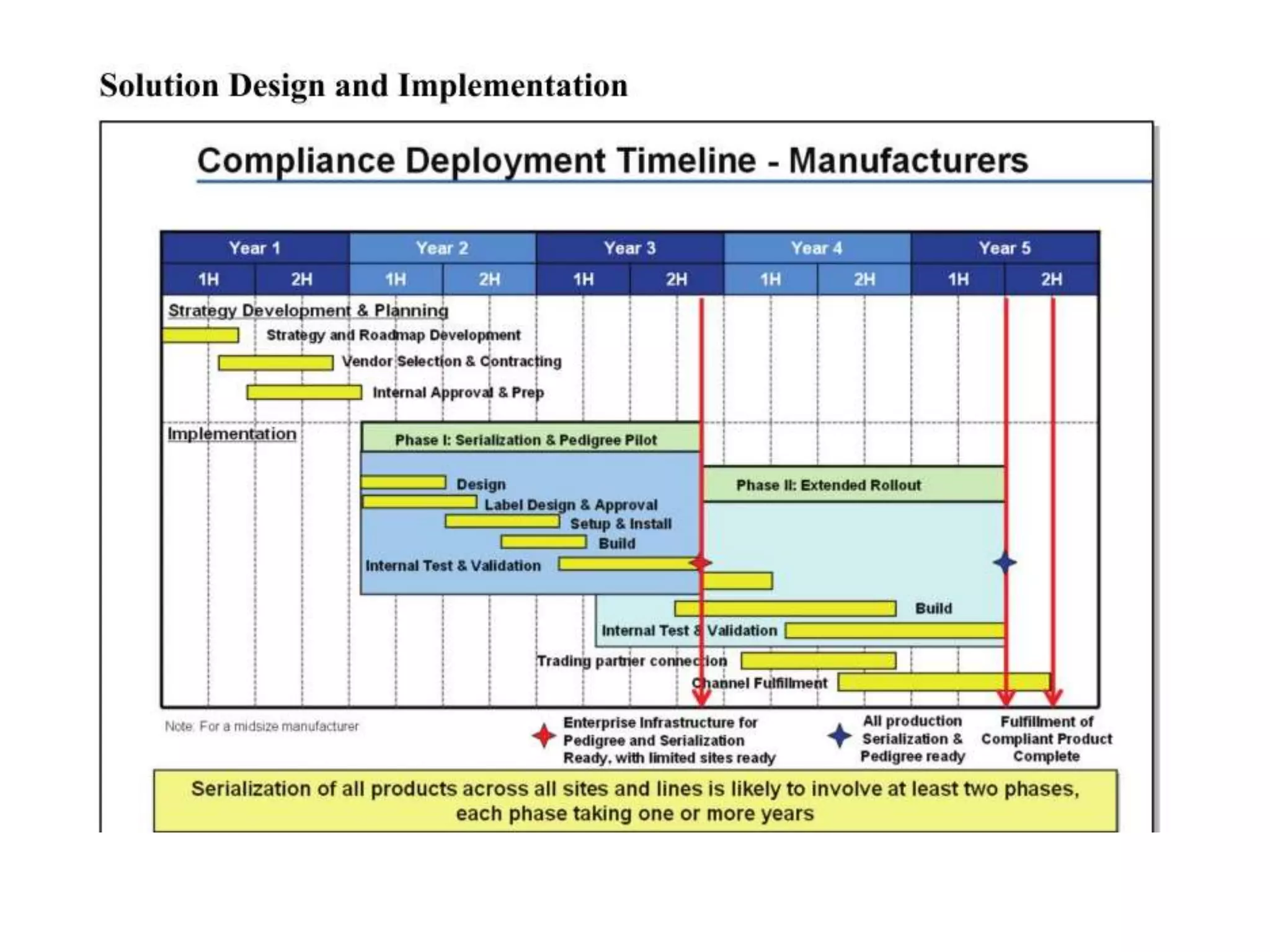
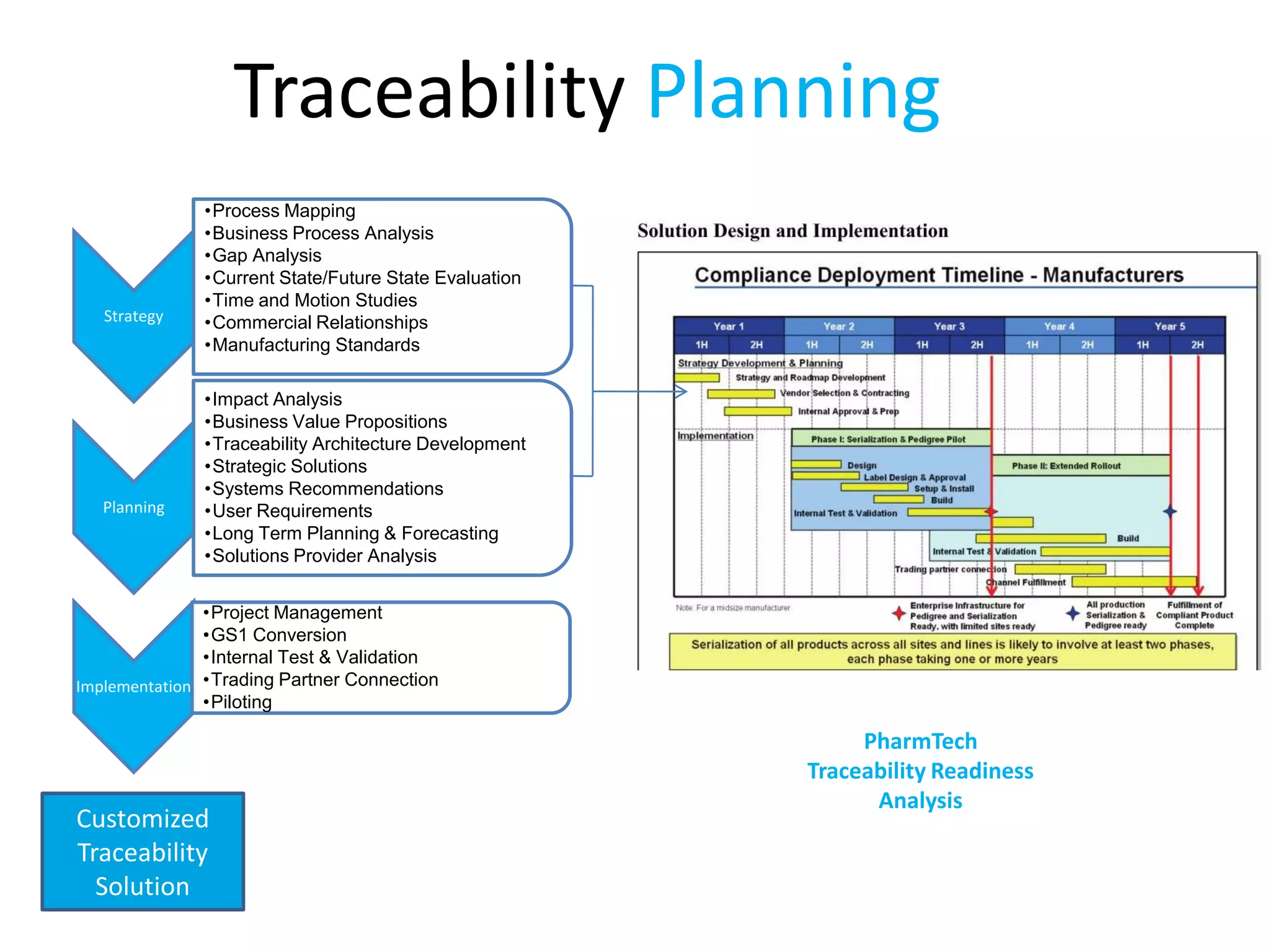
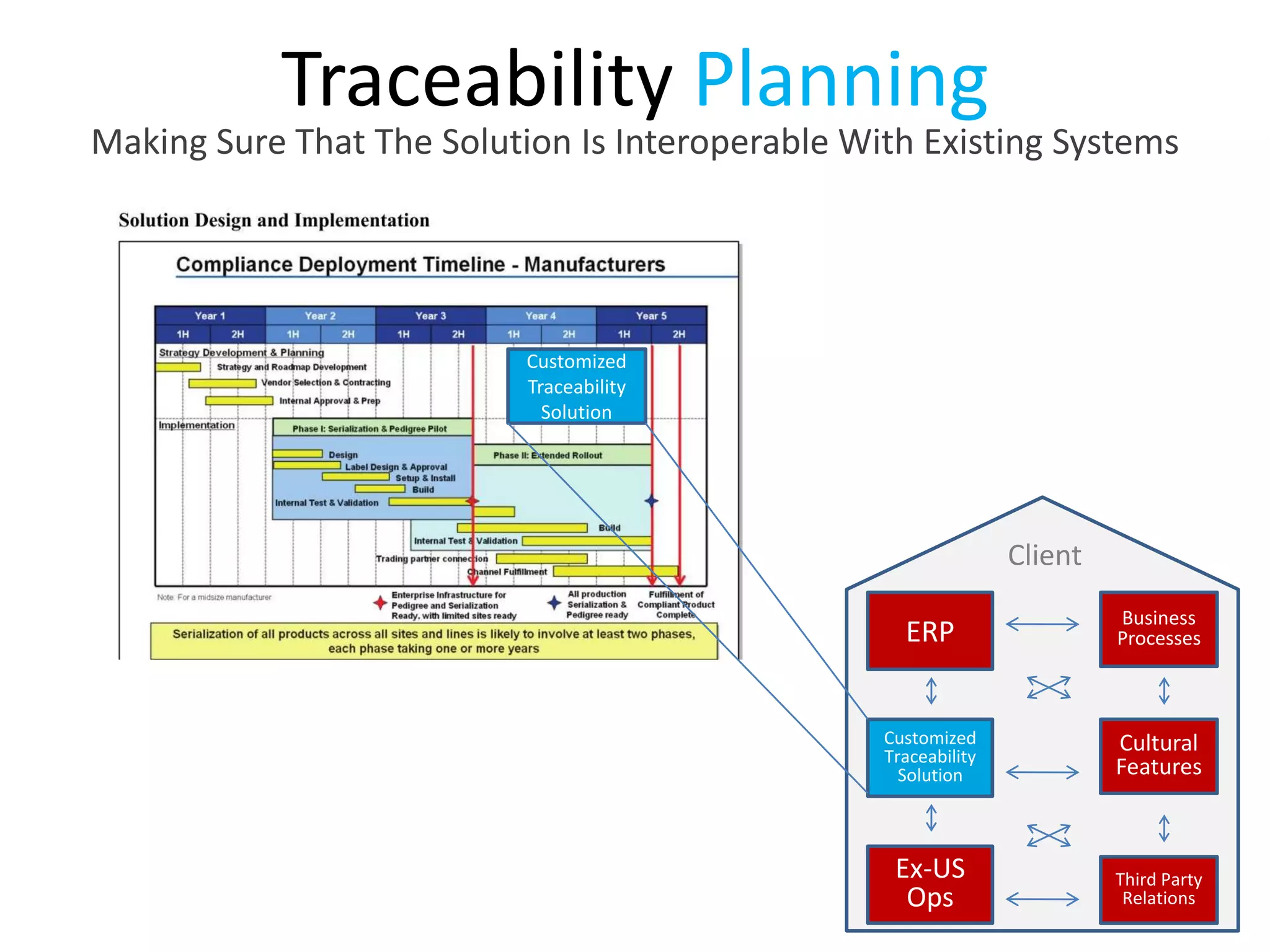
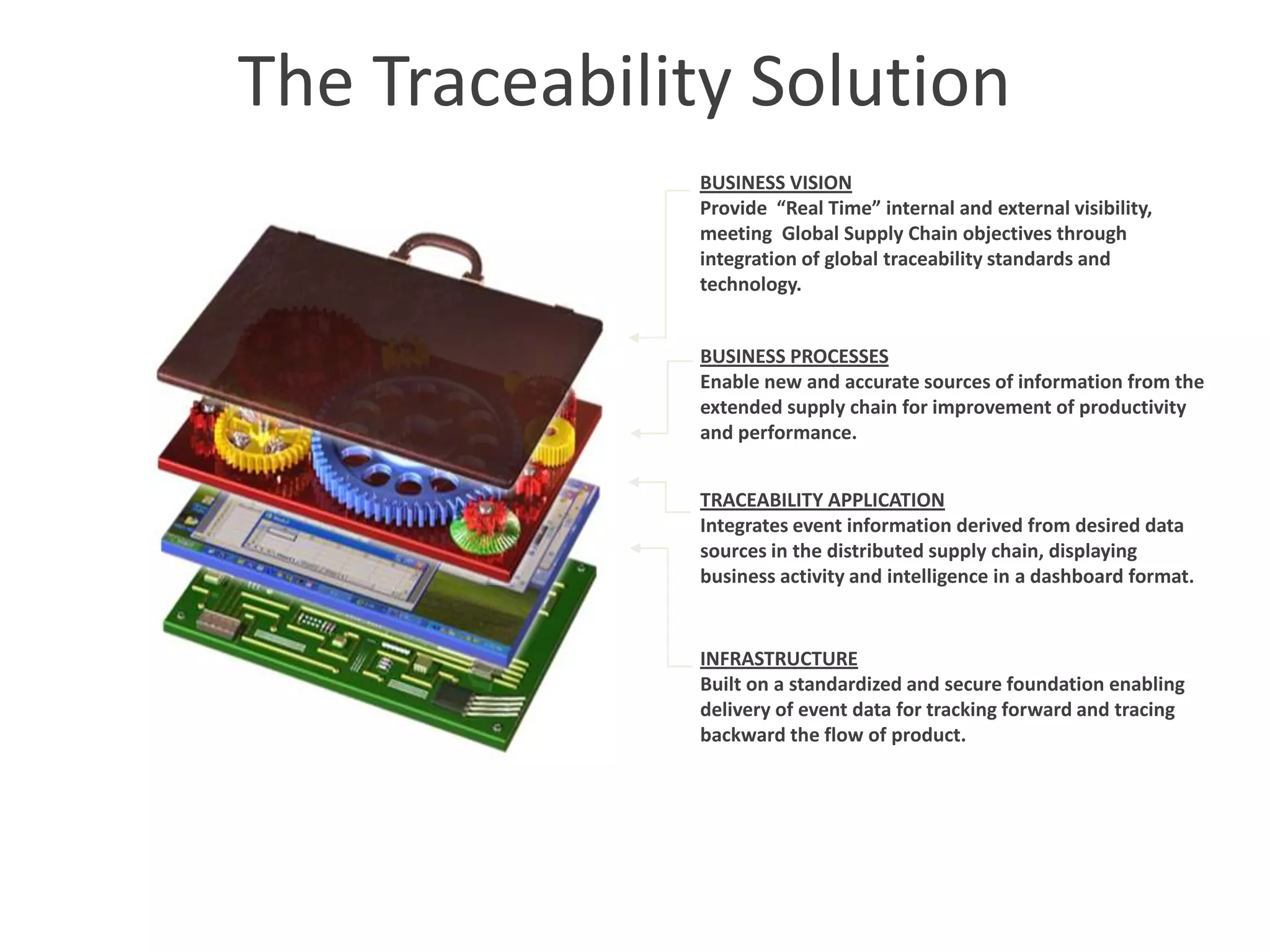
PharmTech is a company that provides track and trace and serialization solutions for pharmaceutical and medical device clients. They have experience implementing traceability solutions globally since 2007. Their services include assessing business needs, developing traceability strategies, planning implementations, and ensuring solutions deliver ROI through improved supply chain visibility and business process optimization. They take a holistic approach to help clients meet regulatory requirements while gaining additional business benefits.