The document discusses trends in Group 18 (noble gases) of the periodic table. It notes that atomic radius decreases from left to right as effective nuclear charge increases. Noble gases are extremely unreactive since they have a stable full outer electron shell. They have the highest ionization energies within their periods since it takes a lot of energy to remove an electron from their stable configuration. Most noble gases are used for lighting applications.



![Neon
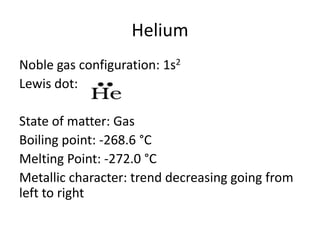
Noble gas configuration: [He] 2s2 2p6
Lewis dot:
State of matter: Gas
Boiling point: -246.1 °C
Melting Point: -248.6 °C
Metallic character: trend decreasing going from
left to right](https://image.slidesharecdn.com/periodictableprojectreal-131111235559-phpapp02/85/Periodic-Table-Project-5-320.jpg)
![Argon
Noble gas configuration: [Ne] 3s2 3p6
Lewis dot:
State of matter: Gas
Boiling point: -186.0 °C
Melting point: -189.3 °C
Metallic character: trend decreasing going from
left to right](https://image.slidesharecdn.com/periodictableprojectreal-131111235559-phpapp02/85/Periodic-Table-Project-6-320.jpg)
![Krypton
Noble gas configuration: [Ar] 3d10 4s2 4p6
Lewis dot:
State of matter: Gas
Boiling point: -153.4 °C
Melting point: -157.2 °C
Metallic character: trend decreasing going from
left to right](https://image.slidesharecdn.com/periodictableprojectreal-131111235559-phpapp02/85/Periodic-Table-Project-7-320.jpg)
![Radon
Noble Gas Configuration: [Xe]6s25d6p6
Lewis dot:
State of Matter: Gas
Boiling Point: -62°C
Melting Point: -71°C
Metallic Character: trend decreasing going from
left to right](https://image.slidesharecdn.com/periodictableprojectreal-131111235559-phpapp02/85/Periodic-Table-Project-8-320.jpg)


![Cesium
Noble gas configuration: [Xe] 6s1
State of matter: solid
Boiling point: 671 ºC
Melting point: 28.5 ºC
Metallic character: trend decreasing going from
left to right](https://image.slidesharecdn.com/periodictableprojectreal-131111235559-phpapp02/85/Periodic-Table-Project-11-320.jpg)
![Barium
Noble gas configuration: [Xe] 6s2
State of matter: solid
Boiling point: 1897ºC
Melting point: 727ºC
Metallic character: trend decreasing going from
left to right](https://image.slidesharecdn.com/periodictableprojectreal-131111235559-phpapp02/85/Periodic-Table-Project-12-320.jpg)
![Lutetium
Noble gas configuration: [Xe] 6s2 5d1
State of matter: solid
Boiling point: 3402°C
Melting point: 1663°C
Metallic character: trend decreasing going from
left to right](https://image.slidesharecdn.com/periodictableprojectreal-131111235559-phpapp02/85/Periodic-Table-Project-13-320.jpg)
![Hafnium
Noble gas configuration: [Xe] 6s2 5d2
State of matter: solid
Boiling point: 4603°C
Melting point: 2233°C
Metallic character: trend decreasing going from
left to right](https://image.slidesharecdn.com/periodictableprojectreal-131111235559-phpapp02/85/Periodic-Table-Project-14-320.jpg)
![Tantalum
Noble gas configuration: [Xe] 6s2 5d3
State of matter: solid
Boiling point: 5458°C
Melting point: 3017°C
Metallic character: trend decreasing going from
left to right](https://image.slidesharecdn.com/periodictableprojectreal-131111235559-phpapp02/85/Periodic-Table-Project-15-320.jpg)
![Tungsten
Noble gas configuration: [Xe] 6s2 5d4
State of matter: solid
Boiling point: 5555°C
Melting point: 3422°C
Metallic character: trend decreasing going from
left to right](https://image.slidesharecdn.com/periodictableprojectreal-131111235559-phpapp02/85/Periodic-Table-Project-16-320.jpg)
![Rhenium
•
•
•
•
•
Noble gas configuration: [Xe] 6s2 5d5
State of matter: solid
Boiling point: 5596°C
Melting point: 3186°C
Metallic character: trend decreasing going
from left to right](https://image.slidesharecdn.com/periodictableprojectreal-131111235559-phpapp02/85/Periodic-Table-Project-17-320.jpg)
![Osmium
Noble gas configuration: [Xe] 6s2 5d6
State of matter: solid
Boiling point: 5012°C
Melting point: 3033°C
Metallic character: trend decreasing going from
left to right](https://image.slidesharecdn.com/periodictableprojectreal-131111235559-phpapp02/85/Periodic-Table-Project-18-320.jpg)
![Iridium
Noble gas configuration: [Xe] 6s2 5d7
State of matter: solid
Boiling point: 4428°C
Melting point: 2446°C
Metallic character: trend decreasing going from
left to right](https://image.slidesharecdn.com/periodictableprojectreal-131111235559-phpapp02/85/Periodic-Table-Project-19-320.jpg)
![Platinum
Noble Gas Configuration: [Xe]6s25d8
State of Matter: Solid
Boiling Point: 3800°C
Melting Point: 1772°C
Metallic Character: trend decreasing going from
left to right](https://image.slidesharecdn.com/periodictableprojectreal-131111235559-phpapp02/85/Periodic-Table-Project-20-320.jpg)
![Gold
Noble Gas Configuration: [Xe]6s25d9
State of Matter: Solid
Boiling Point: 2000°C
Melting Point: 1062°C
Metallic Character: trend decreasing from left to
right](https://image.slidesharecdn.com/periodictableprojectreal-131111235559-phpapp02/85/Periodic-Table-Project-21-320.jpg)
![Mercury
Noble Gas Configuration: [Xe]6s25d10
State of Matter: Liquid
Boiling Point: 356.6°C
Melting Point: -38.9°C
Metallic Character: trend decreasing from left to
right](https://image.slidesharecdn.com/periodictableprojectreal-131111235559-phpapp02/85/Periodic-Table-Project-22-320.jpg)
![Thallium
Noble Gas Configuration: [Xe]6s25d106p1
State of Matter: Solid
Boiling Point: 1473°C
Melting Point: 304°C
Metallic Character: trend decreasing from left to
right](https://image.slidesharecdn.com/periodictableprojectreal-131111235559-phpapp02/85/Periodic-Table-Project-23-320.jpg)
![Lead
Noble Gas Configuration: [Xe]6s25d106p2
State of Matter: Lead
Boiling point: 1755°C
Melting Point: 327°C
Metallic Character: trend decreasing from left to
right](https://image.slidesharecdn.com/periodictableprojectreal-131111235559-phpapp02/85/Periodic-Table-Project-24-320.jpg)
![Bismuth
Noble Gas Configuration: [Xe]6s25d106p3
State of Matter: Solid
Boiling Point: 1420°C
Melting Point: 271°C
Metallic Character: trend decreasing from left to
right](https://image.slidesharecdn.com/periodictableprojectreal-131111235559-phpapp02/85/Periodic-Table-Project-25-320.jpg)
![Polonium
Noble Gas Configuration: [Xe]6s25d106p4
State of Matter: Solid
Boiling Point: 962°C
Melting Point: 254°C
Metallic Character: trend decreasing from left to
right](https://image.slidesharecdn.com/periodictableprojectreal-131111235559-phpapp02/85/Periodic-Table-Project-26-320.jpg)
![Astatine
Noble Gas Configuration: [Xe]6s25d106p5
State of Matter: Solid
Boiling Point: 337°C (estimate)
Melting Point: 302°C
Metallic Character: trend decreasing from left to
right](https://image.slidesharecdn.com/periodictableprojectreal-131111235559-phpapp02/85/Periodic-Table-Project-27-320.jpg)