This document provides an overview of the perforated patch-clamp technique, including:
- The rationale for using perforated patch-clamp is to overcome the dialysis of intracellular components that occurs with traditional whole-cell recording.
- Common perforants like nystatin and amphotericin B form small pores that allow electrical access while preventing diffusion of larger molecules.
- Gramicidin is also used and has the advantage of preserving intracellular chloride concentration.
- Step-by-step guidance is given for performing recordings with nystatin, amphotericin B, and gramicidin. Progress of perforation is monitored by observing changing current responses to voltage pulses.


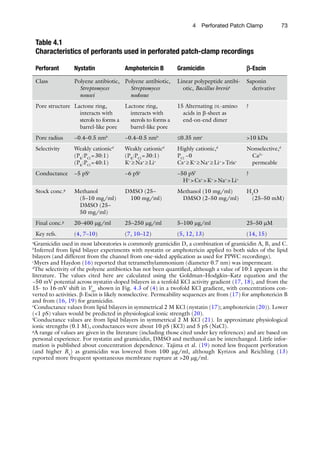

![76 H. Ishibashi et al.
3. Both polyene antibiotics and gramicidin at the tip of the patch
pipette may impair the initial GW seal formation; consequently,
prefilling the tip of the pipette by brief immersion into an anti-
biotic-free solution may be necessary. The optimal time for
pipette tip immersion depends on multiple factors, including
pipette shape and diameter [larger tips require less filling time,
smaller-tip pipettes (~ > 5 MW) may not need filling], the per-
forant concentration used (a lower concentration requires little
or no filling), and the time required between filling and sealing
(with slower procedures requiring more tip filling). The time
can be determined empirically, aided by microscopic inspec-
tion of tip geometries and filling heights. In our experience
with filament-free borosilicate glass capillaries of 3–7 MW and
with short and blunt tapers, filling for about 2 s is optimal. The
remainder of the pipette is back-filled with the internal pipette
solution containing the perforant. Small air bubbles at the tip
are rapidly and easily removed by tapping the pipette shaft with
the index finger.
4. The patch pipette is secured in the pipette holder and dipped
into the external solution in the recording chamber and guided
toward the cell as rapidly as possible, before the antibiotic
diffuses to the tip. No positive pressure is applied during the
approach. After gently pushing the pipette tip onto the cell
membrane, causing a slight increase of the pipette resistance,
gentle negative pressure/suction is applied to obtain the GW
seal (Fig. 4.1b). Canceling the fast capacitive transients helps
with subsequent monitoring of perforation.
5. The pipette potential is subsequently typically held between
−40 and −70 mV, gradually approaching the cell’s resting
membrane potential as perforation proceeds. The progress of
perforation is monitored by visualizing at high temporal reso-
lution the current response to repetitive hyperpolarizing
voltage pulses of about 5–10 mV (DV) (Fig. 4.1b). As the access
resistance (Ra
) decreases and electrical contact with the cell
improves, a current transient due to the cell membrane capaci-
tance (Cm
) is observed. The amplitude of this current transient
is given by DV/Ra
(hence, the current response increases as
perforation proceeds), and the decay time constant is given by
Cm
× Ra
(hence, the current decay gets sharper/faster as perfo-
ration proceeds). The values of Cm
and Ra
can be quantified by
analyzing the current transient or read from the capacitance
and series resistance cancelation dials off the patch-clamp
amplifier. Recordings can commence once the Ra
has stabilized
at a suitable value and then is compensated for. We routinely
obtain stable Ra
< 30 MW by 30 min. Ra
should be monitored
and adjusted during the experiment, and data should be used
only during periods when this value is relatively stable.](https://image.slidesharecdn.com/patchclamptechniques-130519234151-phpapp01/85/Patch-clamp-techniques-6-320.jpg)

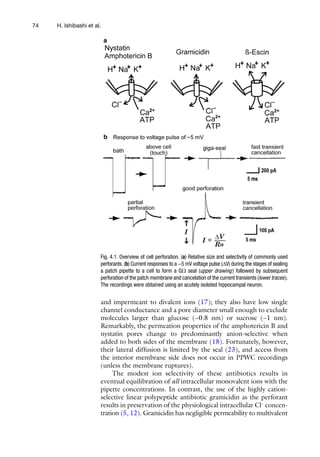
![794 Perforated Patch Clamp
attempts have been made to prevent run-down, including application
of substances to maintain channel phosphorylation and/or protect
against proteolysis, but none of these efforts has been satisfactory.
In contrast, run-down of voltage-activated Ca2+
channels is mark-
edly reduced/delayed using nystatin PPWC recordings (Fig. 4.3).
The stable recording of Ca2+
channel currents has allowed, for
example, detailed pharmacological dissection of the contribution
of different voltage-activated Ca2+
channels in different prepara-
tions (30, 31).
On conventional whole-cell recordings and PPWC recordings
using the polyene antibiotics or b-escin, the intracellular Cl−
con-
centration ([Cl−
]) equilibrates with that in the pipette [Cl−
]. In
contrast, gramicidin PPWC preserves the “normal” intracellular
[Cl−
] and enables one to record the physiological response of
Cl−
-permeant channels; it also allows us to investigate the modulation
of intracellular Cl−
homeostasis. Figure 4.4a compares the response
of ionotropic hippocampal GABA receptors when recorded using
a conventional whole-cell or gramicidin PPWC technique at the
same holding potential (VH
) of −50 mV. The direction of the cur-
rent response is completely different, reflecting the different intra-
cellular [Cl−
] and hence driving force. The physiological range of
intracellular [Cl−
] (~5–30 mM) is less than often used in pipette
solutions in conventional whole-cell recordings (~150 mM). The
gramicidin PPWC technique can be used to measure the intracel-
lular [Cl−
] as shown in Fig. 4.4b. Currents through the anion-
selective ionotropic GABA or glycine receptors are measured at
different holding potentials, and a current–voltage curve can be
4.4.3. GABA and
Glycine Responses
Recorded by the
Gramicidin-Perforated
Patch Recording
Fig. 4.3. Nystatin PPWC mode prevents the run-down of Ca2+
channel currents. Typical traces of high-voltage activated Ca2+
currents recorded from acutely isolated rat intracardiac ganglion cells using conventional (left panel) or PPWC (right panel)
recordings. Current traces obtained at the beginning of the experiment (control) and 30 min later (30 min) are superimposed.](https://image.slidesharecdn.com/patchclamptechniques-130519234151-phpapp01/85/Patch-clamp-techniques-9-320.jpg)
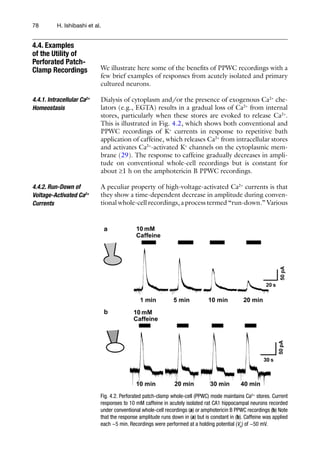
![Fig. 4.4. Physiological g-aminobutyric acid (GABA) and glycine responses and intracellular
[Cl−
] measurements using the gramicidin PPWC technique. (a) GABA-induced outward
and inward currents recorded at a VH
of −50 mV before (left trace) and after (right trace)
rupture of the membrane during a gramicidin PPWC recording. Membrane rupture results
in a much higher intracellular [Cl−
] as the cell equilibrates with the pipette [Cl−
] (~150 mM).
Hence, the GABA response changes from an outward current (representing Cl−
efflux) to
an inward current (Cl−
influx). (b) Glycine-induced currents recorded using the gramicidin
PPWC recording at various VH
s values in cultured spinal cord neurons are measured to
construct a corresponding current–voltage curve from which the Vrev
(arrow) is deter-
mined. The intracellular [Cl−
] is estimated from a derivation of the Nernst equation:
[Cl−
]in
= [Cl−
]out
exp(Vrev
F/RT), where F is Faraday’s constant (96,485 Cmol−1
), R is the gas
constant (8.3145 VCmol−1
K−1
), and T is absolute temperature (293.15 K at 20°C). (c) Such
measurements are used to demonstrate that furosemide causes a reversible increase in
the intracellular [Cl−
]. This is due to direct inhibition of K+
-coupled Cl−
efflux via the neu-
ronal KCC2 transporter. The graph plots the mean ± SEM from five experiments.](https://image.slidesharecdn.com/patchclamptechniques-130519234151-phpapp01/85/Patch-clamp-techniques-10-320.jpg)
![814 Perforated Patch Clamp
plotted. If using ramp voltage responses, control currents in the
absence of GABA or glycine should be subtracted from those in
the presence of GABA or glycine. The X axis intercept of this curve
(the reversal potential, Vrev
) gives the equilibrium potential where
the driving force due to the membrane voltage cancels out that
from the concentration gradient. This is expressed mathematically
by the Nernst equation, from which the intracellular [Cl−
] can be
estimated (Fig. 4.4b). Precise quantification of Vrev
and intracellu-
lar [Cl−
] requires consideration of all permeant ion species, activity
coefficients, and liquid junction potentials (32); the latter can be
up to ~10 mV or more if using larger ions in the pipette solution.
These recordings should use a K+
-based solution in the pipette, as
the intracellular [Cl−
] is sensitive to transmembrane K+
(and, to a
lesser extent, Na+
) gradients, and some other cations (e.g., Cs+
) can
significantly inhibit some of the key Cl−
transporters (33).
Figure 4.4c shows the effect of furosemide, a Cl−
transporter inhib-
itor, on the intracellular [Cl−
] in isolated hippocampal neurons.
The ability to measure the intracellular [Cl−
] by the gramicidin
PPWC technique has facilitated revealing developmental- and
injury-induced changes in intracellular Cl−
homeostasis (34, 35).
This chapter has highlighted some of the benefits of the PPWC
technique, but there are also some drawbacks potential users need
to consider. First, PPWC recordings are much more time-consuming
than conventional whole-cell recordings: fresh perforant-containing
pipette solutions are required, and there is a wait (often ³30 min)
for perforation and stabilization to occur. Second, only under the
most optimal conditions can access/series resistances similar to
those under conventional whole-cell recordings be obtained.
Usually they are much higher and one must (as with conventional
whole-cell recordings) be aware of the voltage errors and filtering
effects of this access resistance. A voltage offset (=pipette current ×
Ra
) must be subtracted from VH
, and the −3 dB of the filtering
effect = 1/[2p × Ra
× Cm
]. Series resistance compensation can reduce
these errors. Finally, one must also be aware of a Donnan potential
between the VH
and the real membrane potential that arises as the
large anions in the cell cannot equilibrate with the patch pipette
solution. As discussed by Horn and Marty (4), this may be as high
as ~10 mV with a KCl pipette solution. This potential can be
reduced by including large impermeant ions in the pipette, but it is
difficult to measure or estimate it precisely. Hence in PPWC recordings,
one must be aware of potential inaccuracies in citing absolute
voltages associated with current responses (e.g., Kd
for activation
or inactivation).
4.5. Drawbacks
of the Perforated
Patch-Clamp
Technique](https://image.slidesharecdn.com/patchclamptechniques-130519234151-phpapp01/85/Patch-clamp-techniques-11-320.jpg)

