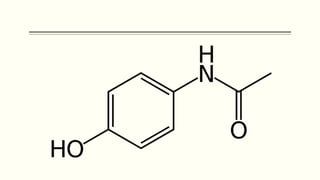
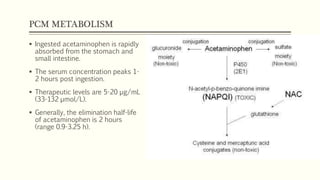
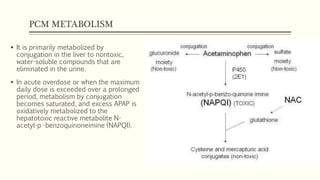
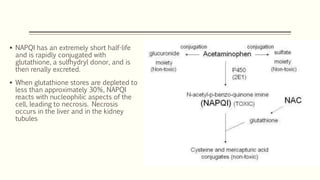
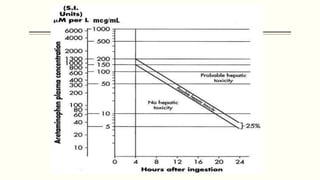
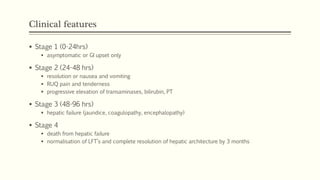
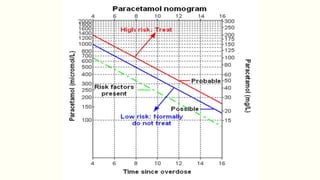
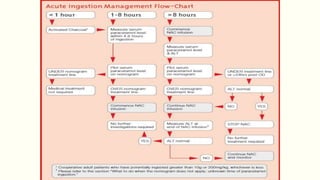
A 35-year-old Indian housewife presented to the hospital 2 hours after intentionally ingesting 20 tablets of paracetamol (acetaminophen) due to family problems and suicidal thoughts. Her physical exam was unremarkable except for tachycardia. Laboratory tests and management for paracetamol overdose were recommended based on the risk of liver toxicity and failure from metabolism of excess amounts into a reactive compound depleted by glutathione stores. Treatment with N-acetylcysteine was indicated based on the timing and amount of ingestion to prevent hepatotoxicity.