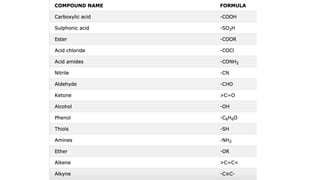
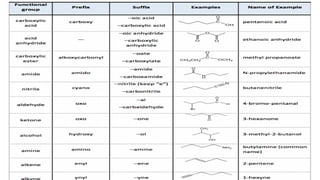
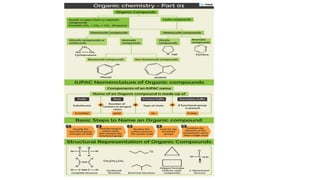
The document provides an overview of the classification and nomenclature of organic compounds, categorizing them into acyclic (open-chain) and cyclic (closed-chain) compounds. It details the IUPAC nomenclature rules for naming alkanes, alkenes, and alkynes, as well as compositional, substitutive, and additive nomenclature methods. The conclusion highlights the significance and complexity of organic compounds in chemistry, emphasizing their carbon-hydrogen bonding.

![CLASSIFICATION OF ORGANIC COMPOUND;
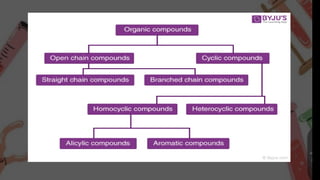
THE ORGANIC COMPOUND BROADLY CLASSIFIED INTO TWO CATEGORIES;
• ACYCLIC COMPOUND OR OPEN CHAIN COMPOUND
• CYCLIC COMPOUND OR CLOSED CHAIN COMPOUND
ACYCLIC COMPOUND OR OPEN CHAIN COMPOUND
• The very first classification of organic compounds is open-chain organic compounds. The organic
compounds whose structural formula is straight are open-chain organic compounds. Open chain
organic compounds are also known as acyclic compounds. Acyclic compounds are those compounds
whose structural formula is not cyclic. For instance: methane, ethane, acetone, methanol etc.
• Open chain compounds (aliphatic compounds) can be classified broadly into two categories which
such as:
• Saturated Compounds[Don’t have double or triple bond between carbon and hydrogen e.g.Alkaens]
• Unsaturated Compounds[have double or triple bond between carbon and hydrogen
e.g.Alkene,Alkynes]](https://image.slidesharecdn.com/chempres-250104060326-08b9d426/85/ORGANIC-CHEMISTRY-NOMENCLEATURE-CLASSIFICATION-2-320.jpg)
![CLOSED CHAIN COMPOUND ;
• Organic compounds are also classified as closed chain organic compounds. The compound in which
carbon is enclosed within a closed chain is known as a closed chain organic compound. Closed chain
organic compounds are also known as cyclic compounds or ring compounds.
• Cyclic compounds are those compounds in which the carbon chain forms a cycle or ring. Such
compounds can also be referred to as ring compounds.
• Cyclic compounds can be classified into two categories which are-
• Homocycliccompounds
• Heterocyclic compounds
* Homocyclic compounds are those cyclic compounds that are purely composed of a carbon
chain. No other functional group is attached to the ring. Such compounds are known as
homocyclic compounds. Homocyclic compounds are nonpolar because they are only
composed of carbon and hydrogen atoms. For instance: cyclo-propane, cyclo-butane etc.
• Homocyclic compounds are further divided into the following categories:
• Alicyclic compounds[ Don’t have double or triple bond between carbon and hydrogen ]
• Aromatic compounds[ Have double or triple bond between carbon and hydrogen ]](https://image.slidesharecdn.com/chempres-250104060326-08b9d426/85/ORGANIC-CHEMISTRY-NOMENCLEATURE-CLASSIFICATION-3-320.jpg)




![• 3. Additive Nomenclature
• This method was formulated primarily for its applications in
the nomenclature of coordination compounds. It has a wide range
of applications. An example for such nomenclature can be
observed in the name Penta-ammine-chloro-cobalt(III) chloride
used to describe the coordination compound given by the chemical
formula [CoCl(NH3)5]Cl2.
• “The prefix ‘chloro’ corresponds to a Chloride, whereas the prefix
‘chlorido’ corresponds to the ligand.”
• An example of this nomenclature can be observed in the name tri-
chlorido-phosphorus which is used to describe the compound with
the formula PCl3](https://image.slidesharecdn.com/chempres-250104060326-08b9d426/85/ORGANIC-CHEMISTRY-NOMENCLEATURE-CLASSIFICATION-11-320.jpg)