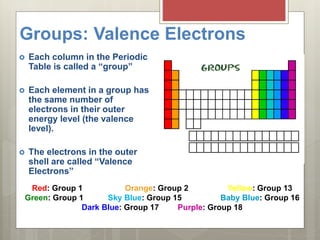
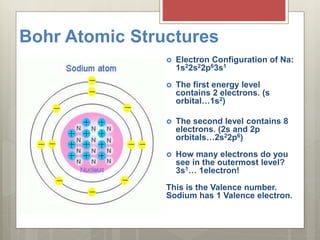
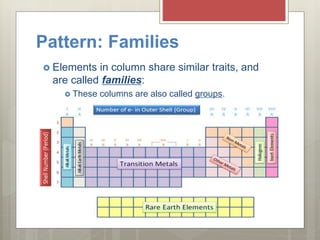
This document discusses the organization and patterns of the periodic table. It explains that each row of the periodic table is called a period, corresponding to an atomic energy level. Elements in the same column, or group, have the same number of valence electrons in their outer shell. Valence electrons are involved in bond formation. Elements in the same family share chemical properties and tend to gain or lose the same number of electrons to achieve stable electron configurations forming ions.